Assessment of Biocide Impacts on Life Support (LS) and Extravehicular Activity (EVA) Architectures
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Effects on Bromine, Chlorine and SO2 Under Air Firing and Oxy-Fuel
GAS-PHASE MERCURY OXIDATION: EFFECTS OF BROMINE, CHLORINE AND SO2 UNDER AIR FIRING AND OXY-FUEL CONDITIONS, EXPERIMENTAL AND MODELING STUDY by Paula Andrea Buitrago A dissertation submitted to the faculty of The University of Utah in partial fulfillment of the requirements for the degree of Doctor of Philosophy Department of Chemical Engineering The University of Utah August 2011 Copyright © Paula Andrea Buitrago 2011 All Rights Reserved STATEMENT OF DISSERTATION APPROVAL The dissertation of Paula Andrea Bnitrago has been approved by the following supervisory committee members: Geoffrey D. Silcox , Chair 4/1112011 Date Approved JoAnn S. Lighty , Member 4/1112011 Date Approved Jost Wendt , Member 4/18/2011 bate Approved Connie Senior , Member 4/15/2011 Date Approved Eric G. Eddings , Member 4/1112011 bate Approved and by JoAnn S. Lighty , Chair of ----------------~~~~==~~--------------- the Department of Chemical Engineering and by Charles A. Wight, Dean of The Graduate School. ABSTRACT The mercury in coal is emitted in its elemental state when the coal is burned. As the combustion flue gas cools, reactions under homogeneous and heterogeneous conditions between mercury and species such chlorine, bromine, SOx and NOx can take place. The temperature and concentration of these species determines the extent of mercury oxidation. The main objective of this study was to evaluate the effects of bromine, chlorine, SOx and NOx on gas-phase mercury oxidation reactions in flue gas. This study used a methane-fired, bench-scale reactor and CHEMKIN software for performing kinetic calculations. The model included reaction pathways to account for halogen, mercury, and combustion chemistry. The experimental results showed that chlorine is not effective as a gas-phase oxidant of mercury compared with other halogens such as bromine. -

IODINE Its Properties and Technical Applications
IODINE Its Properties and Technical Applications CHILEAN IODINE EDUCATIONAL BUREAU, INC. 120 Broadway, New York 5, New York IODINE Its Properties and Technical Applications ¡¡iiHiüíiüüiütitittüHiiUitítHiiiittiíU CHILEAN IODINE EDUCATIONAL BUREAU, INC. 120 Broadway, New York 5, New York 1951 Copyright, 1951, by Chilean Iodine Educational Bureau, Inc. Printed in U.S.A. Contents Page Foreword v I—Chemistry of Iodine and Its Compounds 1 A Short History of Iodine 1 The Occurrence and Production of Iodine ....... 3 The Properties of Iodine 4 Solid Iodine 4 Liquid Iodine 5 Iodine Vapor and Gas 6 Chemical Properties 6 Inorganic Compounds of Iodine 8 Compounds of Electropositive Iodine 8 Compounds with Other Halogens 8 The Polyhalides 9 Hydrogen Iodide 1,0 Inorganic Iodides 10 Physical Properties 10 Chemical Properties 12 Complex Iodides .13 The Oxides of Iodine . 14 Iodic Acid and the Iodates 15 Periodic Acid and the Periodates 15 Reactions of Iodine and Its Inorganic Compounds With Organic Compounds 17 Iodine . 17 Iodine Halides 18 Hydrogen Iodide 19 Inorganic Iodides 19 Periodic and Iodic Acids 21 The Organic Iodo Compounds 22 Organic Compounds of Polyvalent Iodine 25 The lodoso Compounds 25 The Iodoxy Compounds 26 The Iodyl Compounds 26 The Iodonium Salts 27 Heterocyclic Iodine Compounds 30 Bibliography 31 II—Applications of Iodine and Its Compounds 35 Iodine in Organic Chemistry 35 Iodine and Its Compounds at Catalysts 35 Exchange Catalysis 35 Halogenation 38 Isomerization 38 Dehydration 39 III Page Acylation 41 Carbón Monoxide (and Nitric Oxide) Additions ... 42 Reactions with Oxygen 42 Homogeneous Pyrolysis 43 Iodine as an Inhibitor 44 Other Applications 44 Iodine and Its Compounds as Process Reagents ... -

NACE Bromine Chemistry Review Paper
25 YEARS OF BROMINE CHEMISTRY IN INDUSTRIAL WATER SYSTEMS: A REVIEW Christopher J. Nalepa Albemarle Corporation P.O. Box 14799 Baton Rouge, LA 70898 ABSTRACT Bromine chemistry is used to great advantage in nature for fouling control by a number of sessile marine organisms such as sponges, seaweeds, and bryozoans. Such organisms produce small quantities of brominated organic compounds that effectively help keep their surfaces clean of problem bacteria, fungi, and algae. For over two decades, bromine chemistry has been used to similar advantage in the treatment of industrial water systems. The past several years in particular has seen the development of several diverse bromine product forms – one-drum stabilized bromine liquids, all-bromine hydantoin solids, and pumpable gels. The purpose of this paper is to review the development of bromine chemistry in industrial water treatment, discuss characteristics of the new product forms, and speculate on future developments. Keywords: Oxidizing biocide, bleach, bromine, bromine chemistry, sodium hypobromite, activated sodium bromide, Bromochlorodimethylhydantoin, Bromochloromethyethylhydantoin, Dibromodi- methylhydantoin,, BCDMH, BCMEH, DBDMH, stabilized bromine chloride, stabilized hypobromite INTRODUCTION Sessile marine organisms generate metabolites to ward off predators and deter attachment of potential micro- and macrofoulants. Sponges, algae, and bryozoans for example, produce a rich variety of bromine-containing compounds that exhibit antifoulant properties (Fig. 1).1,2,3 Scientists are actively studying these organisms to understand how they maintain surfaces that are relatively clean and slime- free.4 Brominated furanones isolated from the red algae Delisea pulchra, for example, have been found to interfere with the chemical signals (acylated homoserine lactones) that bacteria use to communicate with one another to produce biofilms.5,6 This work may eventually lead to more effective control of microorganisms in a number of industries such as industrial water treatment, oil and gas production, health care, etc. -

CHEM 1411 Nomenclature Homework - Answers Part I
1 CHEM 1411 Nomenclature Homework - Answers Part I 1. The following are a list of binary and pseudobinary ionic compounds. Write the name when the formula is given. Write the formula when the name is given. (a) AlCl3 aluminum chloride (k) rubidium oxide Rb2O (b) AuBr3 gold (III) bromide (l) chromium (III) selenide Cr2Se3 (c) Na2S sodium sulfide (m) barium iodide BaI2 (d) Cu3P2 copper (II) phosphide (n) copper (I) fluoride CuF (e) Fe(OH)2 iron (II) hydroxide (o) copper (II) fluoride CuF2 (f) NH4OH ammonium hydroxide (p) strontium cyanide Sr(CN)2 (g) Co(CH3COO)3 cobalt (III) acetate (q) mercury (II) bromide HgBr2 (h) Zn(SCN)2 zinc thiocyanate (r) mercury (I) bromide Hg2Br2 (i) CaCrO4 calcium chromate (s) magnesium permanganate Mg(MnO4)2 (j) K2Cr2O7 potassium dichromate (t) lithium nitride Li3N 2. The following are lists of covalent compounds. Write the name when a formula is given. Write the formula when given a name. (a) CSe2 carbon diselenide (h) dichlorine heptoxide Cl2O7 (b) SF6 sulfur hexafluoride (i) xenon tetrafluoride XeF4 (c) BrF5 bromine pentafluoride (j) carbon monoxide CO (d) P4O10 tetraphosphorous decoxide (k) oxygen O2 (e) Cl2O dichlorine oxide (l) diboron trioxide B2B O3 (f) NH3 ammonia (m) arsenic trifluoride AsF3 (g) N2 dinitrogen or nitrogen (n) diiodine I2 2 3. The following are lists of acids or acid-forming compounds. Write the name when the formula is given. Write the formula when the name is given. (a) H3PO2 hypophosphorous acid (k) hydrogen cyanide HCN (g) (b) H2SO4 sulfuric acid (l) periodic acid HIO4 (c) HClO hypochlorous acid (m) hypochlorous acid HClO (d) H3PO4 phosphoric acid (n) nitric acid HNO3 (e) HBrO4 perbromic acid (o) acetic acid CH3CO2H (f) HIO2 iodous acid (p) chloric acid HClO3 (g) HI (g) hydrogen iodide (q) perbromic acid HBrO4 (h) HI (aq) hydroiodic acid (r) hydrofluoric acid HF (aq) (i) HCN (aq) hydrocyanic acid (s) phosphorous acid H3PO3 (j) HBrO hypobromous acid (t) hydrosulfuric acid H2S (aq) 4. -

EFGS-SOP-137-R02 and CV-AFS (EPA Method 1631, Rev E)
Document Title: Eurofins Document Reference: Mercury in Water by Oxidation, Purge & Trap EFGS-SOP-137-R02 and CV-AFS (EPA Method 1631, Rev E) Eurofins Document Reference EFGS-SOP-137-R02 Revision 2 Effective Date 6/17/2013 Status Final Historical/Local Document Number FGS-SOP-137.02 Local Document Level Level 3 Local Document Type SOP Local Document Category NA Prepared by Ryan Nelson Reviewed Dave Wunderlich and Patrick Garcia-Strickland and Approved by Revision: 2 Effective Date: 6/17/2013 Page 1 of 20 COMPANY CONFIDENTIAL. All Eurofins Frontier Global Sciences standard operating procedures contain proprietary information and are protected by Washington State Law. Proprietary information must be maintained with the strictest of confidence and must not be used or appropriated to benefit any party without prior written consent from Eurofins Frontier Global Sciences. Document Title: Eurofins Document Reference: Mercury in Water by Oxidation, Purge & Trap EFGS-SOP-137-R02 and CV-AFS (EPA Method 1631, Rev E) Table of Contents 1 Revision Log: ......................................................................................................................... 4 2 Reference: ............................................................................................................................. 4 3 Cross Reference: ................................................................................................................... 5 4 Purpose: ............................................................................................................................... -

Measuring, Control and Sensor Technology 2018
.FBTVSJOH DPOUSPMBOETFOTPSUFDIOPMPHZ 2018 *TTVFECZ 1SP.JOFOU(NC) *N4DIVINBDIFSHFXBOO )FJEFMCFSH Germany Phone +49 6221 842–0 JOGP!QSPNJOFOUDPN www.prominent.com 5FDIOJDBMDIBOHFTSFTFSWFE "MMQSFWJPVTDBUBMPHVFTBOEQSJDFMJTUTBSFTVQFSTFEFEXJUIUIFSFMFBTFPGUIJTQSPEVDUDBUBMPHVF :PVDBOWJFXPVSHFOFSBMUFSNTBOEDPOEJUJPOTPOPVSIPNFQBHF )FJEFMCFSH +BOVBSZ Product Catalogue Volume 2 1.0.1www.prominent.com Product Catalogue Volume 2 Measuring, Control and Sensor Technology Precision by design Precise sensor technology and high-performance measuring and control technology are the guarantee of process safety when metering liquid media. We deal with it in detail in Chapter 1! Discover a huge range of DULCOTEST® sensors for precise recording of different parameters in real time. The controllers in Chapter 2 will introduce consistent quality into your process. From the simple conversion of measuring signals to controllers optimised for complex, application-specific control tasks - the optimum product for every task awaits you here! Completely assembled measuring and control points are described in Chapter 3. They are designed for the measurement of potable water, cooling water and waste water. The ready-wired plug-and-play modules, with perfectly matched components, are ready for fast and easy installation. Chapter 4 is devoted to the treatment of swimming pool water. The product range DULCODOS® Pool is available for this. These complete panel-mounted systems are available in different models - for private pools to public swimming pools. Ready for you. Anytime, anywhere. ProMinent is close to hand no matter where you are: 55 dedicated sales, production and service companies guarantee service and availability in close proximity to our customers. For many years this has meant a local presence for our customers in over 100 countries. Our sales team will be happy to be of assistance should you have any questions about metering technology or water treatment. -

Chemical Toxicology File
L1618Ch10Frame Page 211 Tuesday, August 13, 2002 5:47 PM CHAPTER 10 Toxic Elements 10.1 INTRODUCTION It is somewhat difficult to define what is meant by a toxic element. Some elements, such as white phosphorus, chlorine, and mercury, are quite toxic in the elemental state. Others, such as carbon, nitrogen, and oxygen, are harmless as usually encountered in their normal elemental forms. But, with the exception of those noble gases that do not combine chemically, all elements can form toxic compounds. A prime example is hydrogen cyanide. This extremely toxic compound is formed from three elements that are nontoxic in the uncombined form, and produce compounds that are essential constituents of living matter, but when bonded together in the simple HCN molecule constitute a deadly substance. The following three categories of elements are considered here: • Those that are notable for the toxicities of most of their compounds • Those that form very toxic ions • Those that are very toxic in their elemental forms Elements in these three classes are discussed in this chapter as toxic elements, with the qualification that this category is somewhat arbitrary. With a few exceptions, elements known to be essential to life processes in humans have not been included as toxic elements. 10.2 TOXIC ELEMENTS AND THE PERIODIC TABLE It is most convenient to consider elements from the perspective of the periodic table, which is shown in Figure 1.3 and discussed in Section 1.2. Recall that the three main types of elements, based on their chemical and physical properties as determined by the electron configurations of their atoms, are metals, nonmetals, and metalloids. -
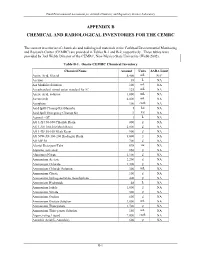
Appendix B Chemical and Radiological Inventories for the Cemrc
Final Environmental Assessment for Actinide Chemistry and Repository Science Laboratory APPENDIX B CHEMICAL AND RADIOLOGICAL INVENTORIES FOR THE CEMRC The current inventories of chemicals and radiological materials at the Carlsbad Environmental Monitoring and Research Center (CEMRC) are provided in Tables B-1 and B-2, respectively. These tables were provided by Joel Webb, Director of the CEMRC, New Mexico State University (Webb 2002). Table B-1. Onsite CEMRC Chemical Inventory Chemical Name Amount Units SARA Limit Acetic Acid, Glacial 5,400 mL NAa Acetone 38 L NA AA Modifier Solution 100 mL NA AccuStandard mixed anion standard for IC 125 mL NA Acetic Acid, solution 1,000 mL NA Acetonitrile 4,000 mL NA Acetylene 100 cu.ft. NA Acid Spill Cleanup Kit (Hazorb) 1 kit NA Acid Spill Emergency Cleanup Kit 3 kit NA Aerosol - OT 1 L NA AG 1-X4 50-100 Chloride Resin 800 g NA AG 1-X8 100-200 Mesh Resin 2,000 g NA AG 1-X8 50-100 Mesh Resin 900 g NA AG 50W-X8 100-200 Hydrogen Resin 3,000 g NA AG MP 50 700 g NA Alcojet Detergent/Tabs 698 oz NA Alumina, activated 850 g NA Aluminum Nitrate 2,100 g NA Ammonium Acetate 2,200 g NA Ammonium Chloride 1,300 g NA Ammonium Chloride Solution 300 mL NA Ammonium Citrate 100 g NA Ammonium hydrogenoxalate, hemihydrate 400 g NA Ammonium Hydroxide 48 L NA Ammonium Iodide 1,000 g NA Ammonium Nitrate 500 g NA Ammonium Oxalate 600 g NA Ammonium Oxalate Solution 2,000 mL NA Ammonium Thiocyanate 1,700 g NA Ammonium Thiocyanate Solution 150 mL NA Argon, refrig. -
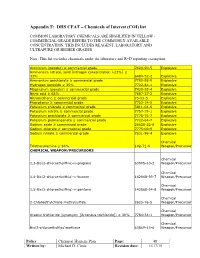
Appendix F: DHS CFAT – Chemicals of Interest (COI) List
Appendix F: DHS CFAT – Chemicals of Interest (COI) list COMMON LABORATORY CHEMICALS ARE HIGHLITED IN YELLOW - COMMERCIAL GRADE REFERS TO THE COMMONLY AVAILABLE CONCENTRATION, THIS INCLUDES REAGENT, LABORATORY AND ULTRAPURE OR HIGHER GRADES Note: This list excludes chemicals under the laboratory and R+D reporting exemption. Aluminum (powder) ≥ commercial grade 7429-90-5 Explosive Ammonium nitrate, solid [nitrogen concentration >23%] ≥ 33% 6484-52-2 Explosive Ammonium perchlorate ≥ commercial grade 7790-98-9 Explosive Hydrogen peroxide ≥ 35% 7722-84-1 Explosive Magnesium (powder) ≥ commercial grade 7439-95-4 Explosive Nitric acid ≥ 68% 7697-37-2 Explosive Nitromethane ≥ commercial grade 75-52-5 Explosive Phosphorus ≥ commercial grade 7723-14-0 Explosive Potassium chlorate ≥ commercial grade 3811-04-9 Explosive Potassium nitrate ≥ commercial grade 7757-79-1 Explosive Potassium perchlorate ≥ commercial grade 7778-74-7 Explosive Potassium permanganate ≥ commercial grade 7722-64-7 Explosive Sodium azide ≥ commercial grade 26628-22-8 Explosive Sodium chlorate ≥ commercial grade 7775-09-9 Explosive Sodium nitrate ≥ commercial grade 7631-99-4 Explosive Chemical Triethanolamine ≥ 80% 102-71-6 Weapon/Precursor CHEMICAL WEAPON/PRECURSORS Chemical 1,3-Bis(2-chloroethylthio)-n-propane 63905-10-2 Weapon/Precursor Chemical 1,4-Bis(2-chloroethylthio)-n-butane 142868-93-7 Weapon/Precursor Chemical 1,5-Bis(2-chloroethylthio)-n-pentane 142868-94-8 Weapon/Precursor Chemical 2-Chloroethylchloro-methylsulfide 2625-76-5 Weapon/Precursor Chemical Arsenic trichloride -

Language of Chemistry 6
CHAPTER Language of Chemistry 6 Section 6.1 Classification of Compounds 2. Compound Classification Compound Classification (a) NH3 binary molecular (b) Fe2O3 binary ionic (c) BaSO4 ternary ionic (d) H2SO4(aq) ternary acid 4. Ion Classification Ion Classification + 2+ (a) NH4 polyatomic cation (b) Sr monoatomic cation 2– 2– (c) S monoatomic anion (d) SO4 polyatomic anion Section 6.2 Monoatomic Ions 6. Monoatomic Cation Stock System Name (a) Ba2+ barium ion (b) Zn2+ zinc ion (c) Co3+ cobalt(III) ion (d) Cu+ copper(I) ion 8. Monoatomic Cation Chemical Formula (a) lithium ion Li+ (b) silver ion Ag+ (c) iron(III) ion Fe3+ (d) tin(IV) ion Sn4+ 10. Monoatomic Cation Latin System Name (a) Cu+ cuprous ion (b) Fe3+ ferric ion (c) Sn4+ stannic ion (d) Pb4+ plumbic ion 2014 © Pearson Education, Inc. Language of Chemistry 33 12. Monoatomic Cation Chemical Formula 2+ (a) mercurous ion Hg2 (b) mercuric ion Hg2+ 14. Monoatomic Anion Systematic Name (a) O2– oxide ion (b) S2– sulfide ion (c) N3– nitride ion (d) P3– phosphide ion Section 6.3 Polyatomic Ions 16. Oxyanion Systematic Name 2– (a) CO3 carbonate ion – (b) HCO3 hydrogen carbonate ion 2– (c) SO4 sulfate ion – (d) HSO4 hydrogen sulfate ion 18. Polyatomic Ion Chemical Formula – (a) chlorate ion ClO3 – (b) perchlorate ion ClO4 – (c) permanganate ion MnO4 – (d) acetate ion C2H3O2 Section 6.4 Writing Chemical Formulas 20. Constituent Ions Chemical Formula (a) Li+ + Cl– LiCl + 2– (b) 2 Ag + O Ag2O 3+ – (c) Cr + 3 I CrI3 2+ 3– (d) 3 Sn + 2 N Sn3N2 22. -

Nitrogen Tribromide Polar Or Nonpolar
Nitrogen tribromide polar or nonpolar Continue Nitrogen Tribromid Names IUPAC Name Nitrogen Tribromid Identifiers CAS Number 15162-90-0 3D Model (JSmol) Interactive Image ChemSpider 20480821 PubChem CID 3082084 CompTox Dashboard (EPA) DTXSID901648 In22 InChI InChIBrH.N/h3'1H;/p-3 SMILES N(Br)(Br)Br Properties Chemical Formula NBr3 Molar Mass 253.7187 g/mol Appearance Deep-red solid melting point explodes at -100 degrees Celsius, except when otherwise noted, the data is given for materials in their standard state (at 25 degrees Celsius), 100 kPa). Infobox links nitrogen tribromid is a chemical compound with the NBr3 formula. It is extremely explosive in its purest form, even at 100 degrees Celsius, and was not isolated until 1975. It's deep-red and volatile solid. The drug NBr3 was first prepared by the reaction of bistrimetligrilbramamin (bis (trimethylsil)amin bromide) with bromine monochloride (with trimethylylyl chloride as a by-product) at 87 degrees on the following equations: (Me3Si)2NBr2 BrCl → NBr3 and 2 Me3SiCl, Where Me is He instantly reacts with ammonia in a dichloromethane solution at 87 degrees Celsius to give NBrH2. Links - Lide, David R. (1998), Handbook on Chemistry and Physics (87 Ed.), Boca-Raton, Florida: CRC Press, p. 4-73, ISBN 0-8493-0594-2 Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of elements (2nd st. Butterworth-Keinmann. page 439. ISBN 978-0-08-037941-8. vteSalts and covalent derivatives of nitrid ion NH3N2H4 He (N2)11 Li3N Be3N2 BN β-C3N4g- C3N4CxNy N2 NxOy NF3 Ne Na3N Mg3 NN2 AlN Si3N4 PNP3N5 SxNySNS4N4 NCl3 Ar K3N Ca3N2 ScN VN CrNCr2N MnxNy FexNy Ni3N CuN n3N2 GaN Ge3N4 as Se NBr3 Kr Rb3 Yn Sr3N2 yn srn NbN β-Mo2N Tc Ru Rh PdN AgN CdN InN Sb Te NI3 Xe Cs3N Ba3N2 Hf3N4 TaN WN Re Os Au Hg3N2 TlN Pb BiN Po At At Rn Fr3N Ra3N2 Rf Db Sg Bh Hs Mt D rg Cn Nh Fl Mc Lv Ts Og s La CeN Pr Nd Pm Sm Eu GdN Tb Dy Er Tm Yb Lu Ac Th Pa UN Np Pu Am Cm Bk Cf Es Fm No Lr Lr Extracted from the Is NBr3 (Nitrogen Tribromid) polar or non-polar? NBr3 (Nitrogen Tribromid) is a polar I'll tell you the polar or nonpolar list below. -

12) United States Patent 10) Patent No.: US 7,173,129 B2
US007 173129B2 12) United States Patent 10) Patent No.: US 7,173,1299 9 B2 Worsencroft et al. (45) Date of Patent: Feb. 6, 2007 (54) SULFONAMIDE-SUBSTITUTED CHALCONE 5,811,449 A 9, 1998 Medford et al. DERVATIVES AND THEIR USE TO TREAT 5,821,260 A 10/1998 Medford et al. DISEASES 5,846,959 A 12/1998 Medford et al. 5,877,203 A 3, 1999 Medford et al. (75) Inventors: Kimberly J. Worsencroft, Alpharetta, 5,951,841 A 9/1999 Wehlage et al. GA (US); Liming Ni, Duluth, GA (US); 6,046,212 A 4/2000 Zwaagstra et al. Zhihong Ye, Lilburn, GA (US); 6,423,740 B1 7/2002 Bombardelli et al. Charleso, Meng, Alpharetta, GA 6,462,075 B1 10/2002 Bowen et al. (US); M. David Weingarten s 6,608, 101 B1 8/2003 Ni et al. s 2003/0232877 A1 12/2003 Sikorski et al. SWISERus) 2003/0236298 Al 12/2003 Meng et al. SE A. Sikorski Atlanta GA (US) 2004/0048858 A1 3/2004 Sikorski et al. (73) Assignee: Athero Genics, Inc., Alpharetta, GA FOREIGN PATENT DOCUMENTS US (US) EP 307762 3, 1989 (*) Notice: Subject to any disclaimer, the term of this EP O 271 307 1, 1992 patent is extended or adjusted under 35 JP 63.01.0720 T 1986 U.S.C. 154(b) by 360 days. JP O4217621 10, 1990 JP O6092950 9, 1992 (21) Appl. No.: 10/863,115 JP 06116206 10, 1992 JP O733O814 6, 1994 (22) Filed: Jun. 7, 2004 WO WO95/1576O 6, 1995 (65) Prior Publication Data (Continued) US 2005/0049236A1 Mar.