A Table of Rotational Constants of Symmetric Top Molecules Giving Rise
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Effects on Bromine, Chlorine and SO2 Under Air Firing and Oxy-Fuel
GAS-PHASE MERCURY OXIDATION: EFFECTS OF BROMINE, CHLORINE AND SO2 UNDER AIR FIRING AND OXY-FUEL CONDITIONS, EXPERIMENTAL AND MODELING STUDY by Paula Andrea Buitrago A dissertation submitted to the faculty of The University of Utah in partial fulfillment of the requirements for the degree of Doctor of Philosophy Department of Chemical Engineering The University of Utah August 2011 Copyright © Paula Andrea Buitrago 2011 All Rights Reserved STATEMENT OF DISSERTATION APPROVAL The dissertation of Paula Andrea Bnitrago has been approved by the following supervisory committee members: Geoffrey D. Silcox , Chair 4/1112011 Date Approved JoAnn S. Lighty , Member 4/1112011 Date Approved Jost Wendt , Member 4/18/2011 bate Approved Connie Senior , Member 4/15/2011 Date Approved Eric G. Eddings , Member 4/1112011 bate Approved and by JoAnn S. Lighty , Chair of ----------------~~~~==~~--------------- the Department of Chemical Engineering and by Charles A. Wight, Dean of The Graduate School. ABSTRACT The mercury in coal is emitted in its elemental state when the coal is burned. As the combustion flue gas cools, reactions under homogeneous and heterogeneous conditions between mercury and species such chlorine, bromine, SOx and NOx can take place. The temperature and concentration of these species determines the extent of mercury oxidation. The main objective of this study was to evaluate the effects of bromine, chlorine, SOx and NOx on gas-phase mercury oxidation reactions in flue gas. This study used a methane-fired, bench-scale reactor and CHEMKIN software for performing kinetic calculations. The model included reaction pathways to account for halogen, mercury, and combustion chemistry. The experimental results showed that chlorine is not effective as a gas-phase oxidant of mercury compared with other halogens such as bromine. -

Chemical Chemical Hazard and Compatibility Information
Chemical Chemical Hazard and Compatibility Information Acetic Acid HAZARDS & STORAGE: Corrosive and combustible liquid. Serious health hazard. Reacts with oxidizing and alkali materials. Keep above freezing point (62 degrees F) to avoid rupture of carboys and glass containers.. INCOMPATIBILITIES: 2-amino-ethanol, Acetaldehyde, Acetic anhydride, Acids, Alcohol, Amines, 2-Amino-ethanol, Ammonia, Ammonium nitrate, 5-Azidotetrazole, Bases, Bromine pentafluoride, Caustics (strong), Chlorosulfonic acid, Chromic Acid, Chromium trioxide, Chlorine trifluoride, Ethylene imine, Ethylene glycol, Ethylene diamine, Hydrogen cyanide, Hydrogen peroxide, Hydrogen sulfide, Hydroxyl compounds, Ketones, Nitric Acid, Oleum, Oxidizers (strong), P(OCN)3, Perchloric acid, Permanganates, Peroxides, Phenols, Phosphorus isocyanate, Phosphorus trichloride, Potassium hydroxide, Potassium permanganate, Potassium-tert-butoxide, Sodium hydroxide, Sodium peroxide, Sulfuric acid, n-Xylene. Acetone HAZARDS & STORAGE: Store in a cool, dry, well ventilated place. INCOMPATIBILITIES: Acids, Bromine trifluoride, Bromine, Bromoform, Carbon, Chloroform, Chromium oxide, Chromium trioxide, Chromyl chloride, Dioxygen difluoride, Fluorine oxide, Hydrogen peroxide, 2-Methyl-1,2-butadiene, NaOBr, Nitric acid, Nitrosyl chloride, Nitrosyl perchlorate, Nitryl perchlorate, NOCl, Oxidizing materials, Permonosulfuric acid, Peroxomonosulfuric acid, Potassium-tert-butoxide, Sulfur dichloride, Sulfuric acid, thio-Diglycol, Thiotrithiazyl perchlorate, Trichloromelamine, 2,4,6-Trichloro-1,3,5-triazine -

Information to Users
INFORMATION TO USERS This manuscript has been reproduced from the microfilm master. UMI films the text directly from the original or copy submitted. Thus, some thesis and dissertation copies are in typewriter face, while others may be from any type of computer printer. The quality of this reproduction is dependent upon the quality of the copy submitted. Broken or indistinct print, colored or poor quality illustrations and photographs, print bleedthrough, substandard margins, and improper alignment can adversely affect reproduction. In the unlikely event that the author did not send UMI a complete manuscript and there are missing pages, these will be noted. Also, if unauthorized copyright material had to be removed, a note will indicate the deletion. Oversize materials (e.g., maps, drawings, charts) are reproduced by sectioning the original, beginning at the upper left-hand corner and continuing from left to right in equal sections with small overlaps. Each original is also photographed in one exposure and is included in reduced form at the back of the book. Photographs included in the original manuscript have been reproduced xerographically in this copy. Higher quality 6" x 9" black and white photographic prints are available for any photographs or illustrations appearing in this copy for an additional charge. Contact UMI directly to order. University Microfilms International A Bell & Howell Information Company 300 North Zeeb Road. Ann Arbor. Ml 48106-1346 USA 313/761-4700 800/521-0600 Order Number 9505198 T h e 1 /1 1 band of cyanuric fluoride and the A *11 - X *E+ transition of aluminum monobromide Fleming, Patrick E., Ph.D. -

Megalomania's Controversial Chem Lab
Megalomania's Controversial Chem Lab Navigation Welcome to the Controversial Chem Lab. Here at the Chem Lab you » Home can find information on a large number of chemicals that have a » Explosives certain stigma attached to them. Chemicals such as explosives, drugs, and pesticides are vitally important for the survival of our civilization. » Chemical Unfortunately, the scientific elite jealously hoards the knowledge on Weapons using and preparing these chemicals. Adding to the confusion is the » Pharmaceuticals scientific ignorant who fear chemistry and think these chemicals are » Pesticides dangerous. As my chemistry professor used to say about what they think, “chemistry equals bad.” » Precursors The Controversial Chem Lab was created to be a free reference on » Lab Skills how to synthesize chemicals. It is also a virtual laboratory skills » Lab Equipment manual, complete with descriptions on how to conduct laboratories, » Safety and a visual database on many different kinds of laboratory apparatus. While the Chem Lab is written for the non-chemist audience, it does » Rogue Science require a basic understanding of laboratory skills. Of course, all of the » Links information needed to acquire a basic understanding of lab skills is » What’s New included within the site. The Chem Lab even goes the extra mile in providing information on » Contact Me how to synthesize many of the chemicals used in making explosives, » Disclaimer etc. It also provides information on where to acquire certain chemicals » Search this site and apparatus. While all of this information is perfectly legal, it may be against the law in certain areas to prepare some of these chemicals without the proper license. -

EFGS-SOP-137-R02 and CV-AFS (EPA Method 1631, Rev E)
Document Title: Eurofins Document Reference: Mercury in Water by Oxidation, Purge & Trap EFGS-SOP-137-R02 and CV-AFS (EPA Method 1631, Rev E) Eurofins Document Reference EFGS-SOP-137-R02 Revision 2 Effective Date 6/17/2013 Status Final Historical/Local Document Number FGS-SOP-137.02 Local Document Level Level 3 Local Document Type SOP Local Document Category NA Prepared by Ryan Nelson Reviewed Dave Wunderlich and Patrick Garcia-Strickland and Approved by Revision: 2 Effective Date: 6/17/2013 Page 1 of 20 COMPANY CONFIDENTIAL. All Eurofins Frontier Global Sciences standard operating procedures contain proprietary information and are protected by Washington State Law. Proprietary information must be maintained with the strictest of confidence and must not be used or appropriated to benefit any party without prior written consent from Eurofins Frontier Global Sciences. Document Title: Eurofins Document Reference: Mercury in Water by Oxidation, Purge & Trap EFGS-SOP-137-R02 and CV-AFS (EPA Method 1631, Rev E) Table of Contents 1 Revision Log: ......................................................................................................................... 4 2 Reference: ............................................................................................................................. 4 3 Cross Reference: ................................................................................................................... 5 4 Purpose: ............................................................................................................................... -

Report | CAMEO Chemicals | NOAA
Report | CAMEO Chemicals | NOAA http://cameochemicals.noaa.gov/report?key=CH2013 Print Chemical Datasheet 0 HYDROFLUORIC ACID 4 1 Chemical Identifiers UN/NA Number CAS Number CHRIS Code DOT Hazard Label 1790 7664-39-3 HFA CORROSIVE POISON NFPA 704: Red 0 -- Flammability: Will not burn Blue 4 -- Health Hazard: Too dangerous to enter - vapor or liquid Yellow 1 -- Reactivity: Unstable if heated - use normal precautions General Description A colorless fuming mobile aqueous solution with a pungent odor. Density 9.6 lb / gal. Corrosive to metals and tissue. Highly toxic by ingestion and inhalation. Exposure to fumes or very short contact with liquid may cause severe painful burns; penetrates skin to cause deep-seated ulceration that may lead to gangrene. Hazards Reactivity Alerts 1 of 8 7/15/09 12:07 AM Report | CAMEO Chemicals | NOAA http://cameochemicals.noaa.gov/report?key=CH2013 Water-Reactive Air-Reactive Air & Water Reactions Fumes in air. Fumes are highly irritating, corrosive, and poisonous. Generates much heat on dissolution [Merck, 11th ed., 1989]. Heat can cause spattering, fuming, etc. Fire Hazard Non-combustible, substance itself does not burn but may decompose upon heating to produce corrosive and/or toxic fumes. Vapors may accumulate in confined areas (basement, tanks, hopper/tank cars etc.). Substance will react with water (some violently), releasing corrosive and/or toxic gases and runoff. Contact with metals may evolve flammable hydrogen gas. Containers may explode when heated or if contaminated with water. (ERG, 2008) Health Hazard TOXIC; inhalation, ingestion or contact (skin, eyes) with vapors, dusts or substance may cause severe injury, burns or death. -

Supplementary Material To: Assessment of Density Functional Methods for Exciton Binding Energies and Related Optoelectronic Properties
Electronic Supplementary Material (ESI) for RSC Advances. This journal is © The Royal Society of Chemistry 2015 Supplementary Material to: Assessment of Density Functional Methods for Exciton Binding Energies and Related Optoelectronic Properties Jui-Che Lee,† Jeng-Da Chai*‡ and Shiang-Tai Lin*† †Department of Chemical Engineering, National Taiwan University, Taipei 10617, Taiwan. E-mail: [email protected] ‡Department of Physics, Center for Theoretical Sciences, and Center for Quantum Science and Engineering, National Taiwan University, Taipei 10617, Taiwan. E-mail: [email protected] Table S1. Vertical ionization potentials (1) of the 121 molecules studied (pp. 3-6). Table S2. Vertical ionization potentials (2) of the 121 molecules studied (pp. 7-10). Table S3. Vertical electron affinities (1) of the 121 molecules studied (pp. 11-13). Table S4. Vertical electron affinities (2) of the 121 molecules studied (pp. 14-17). Table S5. Vertical electron affinities (3) of the 121 molecules studied (pp. 18-21). Table S6. Fundamental gaps (1) of the 121 molecules studied (pp. 23-26). Table S7. Fundamental gaps (2) of the 121 molecules studied (pp. 27-30). Table S8. Fundamental gaps (3) of the 121 molecules studied (pp. 31-34). Table S9. Optical gaps of the 121 molecules studied (pp. 35-38). Table S10. Exciton binding energies (1) of the 121 molecules studied (pp. 39-42). Table S11. Exciton binding energies (2) of the 121 molecules studied (pp. 43-46). Table S12. Exciton binding energies (3) of the 121 molecules studied (pp. 47-50). 1 Table S13. Statistical errors (in eV) of CCSD and CCSD(T) methods for IP(1) (pp. -
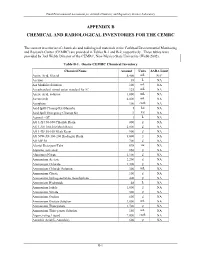
Appendix B Chemical and Radiological Inventories for the Cemrc
Final Environmental Assessment for Actinide Chemistry and Repository Science Laboratory APPENDIX B CHEMICAL AND RADIOLOGICAL INVENTORIES FOR THE CEMRC The current inventories of chemicals and radiological materials at the Carlsbad Environmental Monitoring and Research Center (CEMRC) are provided in Tables B-1 and B-2, respectively. These tables were provided by Joel Webb, Director of the CEMRC, New Mexico State University (Webb 2002). Table B-1. Onsite CEMRC Chemical Inventory Chemical Name Amount Units SARA Limit Acetic Acid, Glacial 5,400 mL NAa Acetone 38 L NA AA Modifier Solution 100 mL NA AccuStandard mixed anion standard for IC 125 mL NA Acetic Acid, solution 1,000 mL NA Acetonitrile 4,000 mL NA Acetylene 100 cu.ft. NA Acid Spill Cleanup Kit (Hazorb) 1 kit NA Acid Spill Emergency Cleanup Kit 3 kit NA Aerosol - OT 1 L NA AG 1-X4 50-100 Chloride Resin 800 g NA AG 1-X8 100-200 Mesh Resin 2,000 g NA AG 1-X8 50-100 Mesh Resin 900 g NA AG 50W-X8 100-200 Hydrogen Resin 3,000 g NA AG MP 50 700 g NA Alcojet Detergent/Tabs 698 oz NA Alumina, activated 850 g NA Aluminum Nitrate 2,100 g NA Ammonium Acetate 2,200 g NA Ammonium Chloride 1,300 g NA Ammonium Chloride Solution 300 mL NA Ammonium Citrate 100 g NA Ammonium hydrogenoxalate, hemihydrate 400 g NA Ammonium Hydroxide 48 L NA Ammonium Iodide 1,000 g NA Ammonium Nitrate 500 g NA Ammonium Oxalate 600 g NA Ammonium Oxalate Solution 2,000 mL NA Ammonium Thiocyanate 1,700 g NA Ammonium Thiocyanate Solution 150 mL NA Argon, refrig. -
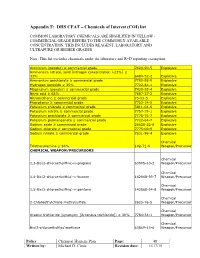
Appendix F: DHS CFAT – Chemicals of Interest (COI) List
Appendix F: DHS CFAT – Chemicals of Interest (COI) list COMMON LABORATORY CHEMICALS ARE HIGHLITED IN YELLOW - COMMERCIAL GRADE REFERS TO THE COMMONLY AVAILABLE CONCENTRATION, THIS INCLUDES REAGENT, LABORATORY AND ULTRAPURE OR HIGHER GRADES Note: This list excludes chemicals under the laboratory and R+D reporting exemption. Aluminum (powder) ≥ commercial grade 7429-90-5 Explosive Ammonium nitrate, solid [nitrogen concentration >23%] ≥ 33% 6484-52-2 Explosive Ammonium perchlorate ≥ commercial grade 7790-98-9 Explosive Hydrogen peroxide ≥ 35% 7722-84-1 Explosive Magnesium (powder) ≥ commercial grade 7439-95-4 Explosive Nitric acid ≥ 68% 7697-37-2 Explosive Nitromethane ≥ commercial grade 75-52-5 Explosive Phosphorus ≥ commercial grade 7723-14-0 Explosive Potassium chlorate ≥ commercial grade 3811-04-9 Explosive Potassium nitrate ≥ commercial grade 7757-79-1 Explosive Potassium perchlorate ≥ commercial grade 7778-74-7 Explosive Potassium permanganate ≥ commercial grade 7722-64-7 Explosive Sodium azide ≥ commercial grade 26628-22-8 Explosive Sodium chlorate ≥ commercial grade 7775-09-9 Explosive Sodium nitrate ≥ commercial grade 7631-99-4 Explosive Chemical Triethanolamine ≥ 80% 102-71-6 Weapon/Precursor CHEMICAL WEAPON/PRECURSORS Chemical 1,3-Bis(2-chloroethylthio)-n-propane 63905-10-2 Weapon/Precursor Chemical 1,4-Bis(2-chloroethylthio)-n-butane 142868-93-7 Weapon/Precursor Chemical 1,5-Bis(2-chloroethylthio)-n-pentane 142868-94-8 Weapon/Precursor Chemical 2-Chloroethylchloro-methylsulfide 2625-76-5 Weapon/Precursor Chemical Arsenic trichloride -

Language of Chemistry 6
CHAPTER Language of Chemistry 6 Section 6.1 Classification of Compounds 2. Compound Classification Compound Classification (a) NH3 binary molecular (b) Fe2O3 binary ionic (c) BaSO4 ternary ionic (d) H2SO4(aq) ternary acid 4. Ion Classification Ion Classification + 2+ (a) NH4 polyatomic cation (b) Sr monoatomic cation 2– 2– (c) S monoatomic anion (d) SO4 polyatomic anion Section 6.2 Monoatomic Ions 6. Monoatomic Cation Stock System Name (a) Ba2+ barium ion (b) Zn2+ zinc ion (c) Co3+ cobalt(III) ion (d) Cu+ copper(I) ion 8. Monoatomic Cation Chemical Formula (a) lithium ion Li+ (b) silver ion Ag+ (c) iron(III) ion Fe3+ (d) tin(IV) ion Sn4+ 10. Monoatomic Cation Latin System Name (a) Cu+ cuprous ion (b) Fe3+ ferric ion (c) Sn4+ stannic ion (d) Pb4+ plumbic ion 2014 © Pearson Education, Inc. Language of Chemistry 33 12. Monoatomic Cation Chemical Formula 2+ (a) mercurous ion Hg2 (b) mercuric ion Hg2+ 14. Monoatomic Anion Systematic Name (a) O2– oxide ion (b) S2– sulfide ion (c) N3– nitride ion (d) P3– phosphide ion Section 6.3 Polyatomic Ions 16. Oxyanion Systematic Name 2– (a) CO3 carbonate ion – (b) HCO3 hydrogen carbonate ion 2– (c) SO4 sulfate ion – (d) HSO4 hydrogen sulfate ion 18. Polyatomic Ion Chemical Formula – (a) chlorate ion ClO3 – (b) perchlorate ion ClO4 – (c) permanganate ion MnO4 – (d) acetate ion C2H3O2 Section 6.4 Writing Chemical Formulas 20. Constituent Ions Chemical Formula (a) Li+ + Cl– LiCl + 2– (b) 2 Ag + O Ag2O 3+ – (c) Cr + 3 I CrI3 2+ 3– (d) 3 Sn + 2 N Sn3N2 22. -

Nitrogen Tribromide Polar Or Nonpolar
Nitrogen tribromide polar or nonpolar Continue Nitrogen Tribromid Names IUPAC Name Nitrogen Tribromid Identifiers CAS Number 15162-90-0 3D Model (JSmol) Interactive Image ChemSpider 20480821 PubChem CID 3082084 CompTox Dashboard (EPA) DTXSID901648 In22 InChI InChIBrH.N/h3'1H;/p-3 SMILES N(Br)(Br)Br Properties Chemical Formula NBr3 Molar Mass 253.7187 g/mol Appearance Deep-red solid melting point explodes at -100 degrees Celsius, except when otherwise noted, the data is given for materials in their standard state (at 25 degrees Celsius), 100 kPa). Infobox links nitrogen tribromid is a chemical compound with the NBr3 formula. It is extremely explosive in its purest form, even at 100 degrees Celsius, and was not isolated until 1975. It's deep-red and volatile solid. The drug NBr3 was first prepared by the reaction of bistrimetligrilbramamin (bis (trimethylsil)amin bromide) with bromine monochloride (with trimethylylyl chloride as a by-product) at 87 degrees on the following equations: (Me3Si)2NBr2 BrCl → NBr3 and 2 Me3SiCl, Where Me is He instantly reacts with ammonia in a dichloromethane solution at 87 degrees Celsius to give NBrH2. Links - Lide, David R. (1998), Handbook on Chemistry and Physics (87 Ed.), Boca-Raton, Florida: CRC Press, p. 4-73, ISBN 0-8493-0594-2 Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of elements (2nd st. Butterworth-Keinmann. page 439. ISBN 978-0-08-037941-8. vteSalts and covalent derivatives of nitrid ion NH3N2H4 He (N2)11 Li3N Be3N2 BN β-C3N4g- C3N4CxNy N2 NxOy NF3 Ne Na3N Mg3 NN2 AlN Si3N4 PNP3N5 SxNySNS4N4 NCl3 Ar K3N Ca3N2 ScN VN CrNCr2N MnxNy FexNy Ni3N CuN n3N2 GaN Ge3N4 as Se NBr3 Kr Rb3 Yn Sr3N2 yn srn NbN β-Mo2N Tc Ru Rh PdN AgN CdN InN Sb Te NI3 Xe Cs3N Ba3N2 Hf3N4 TaN WN Re Os Au Hg3N2 TlN Pb BiN Po At At Rn Fr3N Ra3N2 Rf Db Sg Bh Hs Mt D rg Cn Nh Fl Mc Lv Ts Og s La CeN Pr Nd Pm Sm Eu GdN Tb Dy Er Tm Yb Lu Ac Th Pa UN Np Pu Am Cm Bk Cf Es Fm No Lr Lr Extracted from the Is NBr3 (Nitrogen Tribromid) polar or non-polar? NBr3 (Nitrogen Tribromid) is a polar I'll tell you the polar or nonpolar list below. -

100022-3 Hafnium (1000Μg/Ml in 2% HNO3 + 0.5% HF)
100022-3 Hafnium (1000μg/mL in 2% HNO3 + 0.5% HF) High-Purity Standards Chemwatch Hazard Alert Code: 3 Catalogue number: 100022-3 Issue Date: 06/15/2017 Version No: 3.3 Print Date: 06/15/2017 Safety Data Sheet according to OSHA HazCom Standard (2012) requirements S.GHS.USA.EN SECTION 1 IDENTIFICATION Product Identifier Product name 100022-3 Hafnium (1000μg/mL in 2% HNO3 + 0.5% HF) Synonyms 1000μg/mL Hafnium in 2% HNO3 + 0.5%HF Proper shipping name Corrosive liquid, acidic, inorganic, n.o.s. (contains nitric acid and hydrofluoric acid) Other means of 100022-3 identification Recommended use of the chemical and restrictions on use Relevant identified uses Use according to manufacturer's directions. Name, address, and telephone number of the chemical manufacturer, importer, or other responsible party Registered company name High-Purity Standards Address PO Box 41727 SC 29423 United States Telephone 843-767-7900 Fax 843-767-7906 Website highpuritystandards.com Email Not Available Emergency phone number Association / Organisation INFOTRAC Emergency telephone 1-800-535-5053 numbers Other emergency telephone 1-352-323-3500 numbers SECTION 2 HAZARD(S) IDENTIFICATION Classification of the substance or mixture Acute Toxicity (Oral) Category 4, Acute Toxicity (Dermal) Category 3, Metal Corrosion Category 1, Skin Corrosion/Irritation Category 1A, Serious Eye Classification Damage Category 1 Label elements Hazard pictogram(s) SIGNAL WORD DANGER Hazard statement(s) H302 Harmful if swallowed. H311 Toxic in contact with skin. H290 May be corrosive to metals. H314 Causes severe skin burns and eye damage. Continued... Chemwatch: 9-246917 Page 2 of 10 Issue Date: 06/15/2017 Catalogue number: 100022-3 100022-3 Hafnium (1000μg/mL in 2% HNO3 + 0.5% HF) Print Date: 06/15/2017 Version No: 3.3 Hazard(s) not otherwise specified Not Applicable Precautionary statement(s) Prevention P260 Do not breathe dust/fume/gas/mist/vapours/spray.