Reflex Vertical Gaze and the Medial Longitudinal Fasciculus L
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Optogenetic Fmri Interrogation of Brain-Wide Central Vestibular Pathways
Optogenetic fMRI interrogation of brain-wide central vestibular pathways Alex T. L. Leonga,b, Yong Guc, Ying-Shing Chand, Hairong Zhenge, Celia M. Donga,b, Russell W. Chana,b, Xunda Wanga,b, Yilong Liua,b, Li Hai Tanf, and Ed X. Wua,b,d,g,1 aLaboratory of Biomedical Imaging and Signal Processing, The University of Hong Kong, Pokfulam, Hong Kong SAR, China; bDepartment of Electrical and Electronic Engineering, The University of Hong Kong, Pokfulam, Hong Kong SAR, China; cInstitute of Neuroscience, Key Laboratory of Primate Neurobiology, CAS Center for Excellence in Brain Science and Intelligence Technology, Chinese Academy of Sciences, Shanghai 200031, China; dSchool of Biomedical Sciences, Li Ka Shing Faculty of Medicine, The University of Hong Kong, Pokfulam, Hong Kong SAR, China; eShenzhen Institutes of Advanced Technology, Chinese Academy of Sciences, Shenzhen 518055, China; fCenter for Language and Brain, Shenzhen Institute of Neuroscience, Shenzhen 518057, China; and gState Key Laboratory of Pharmaceutical Biotechnology, The University of Hong Kong, Pokfulam, Hong Kong SAR, China Edited by Marcus E. Raichle, Washington University in St. Louis, St. Louis, MO, and approved March 20, 2019 (received for review July 20, 2018) Blood oxygen level-dependent functional MRI (fMRI) constitutes a multisensory integration process in the vestibular system is op- powerful neuroimaging technology to map brain-wide functions tokinetic nystagmus, whereby visual cues are used to induce in response to specific sensory or cognitive tasks. However, fMRI compensatory reflexive eye movements to maintain a stable gaze mapping of the vestibular system, which is pivotal for our sense of while moving (11, 12). These eye movements involve inputs from balance, poses significant challenges. -

A Network of Genetic Repression and Derepression Specifies Projection
A network of genetic repression and derepression INAUGURAL ARTICLE specifies projection fates in the developing neocortex Karpagam Srinivasana,1, Dino P. Leonea, Rosalie K. Batesona, Gergana Dobrevab, Yoshinori Kohwic, Terumi Kohwi-Shigematsuc, Rudolf Grosschedlb, and Susan K. McConnella,2 aDepartment of Biology, Stanford University, Stanford, CA 94305; bMax-Planck Institute of Immunobiology and Epigenetics, 79108 Freiburg, Germany; and cLawrence Berkeley National Laboratory, Berkeley, CA 94720 This contribution is part of the special series of Inaugural Articles by members of the National Academy of Sciences elected in 2011. Contributed by Susan K. McConnell, September 28, 2012 (sent for review August 24, 2012) Neurons within each layer in the mammalian cortex have stereotypic which results in a suppression of corticothalamic and callosal projections. Four genes—Fezf2, Ctip2, Tbr1, and Satb2—regulate fates, respectively, and axon extension along the corticospinal these projection identities. These genes also interact with each tract (CST). Fezf2 mutant neurons fail to repress Satb2 and Tbr1, other, and it is unclear how these interactions shape the final pro- thus their axons cross the CC and/or innervate the thalamus jection identity. Here we show, by generating double mutants of inappropriately. Fezf2, Ctip2, and Satb2, that cortical neurons deploy a complex In callosal projection neurons, Satb2 represses the expression Ctip2 Bhlhb5 genetic switch that uses mutual repression to produce subcortical of and , leading to a repression of subcerebral fates. Satb2 Tbr1 or callosal projections. We discovered that Tbr1, EphA4, and Unc5H3 Interestingly, we found that promotes expression in fi upper layer callosal neurons, and Tbr1 expression in these neu- are critical downstream targets of Satb2 in callosal fate speci ca- fi tion. -
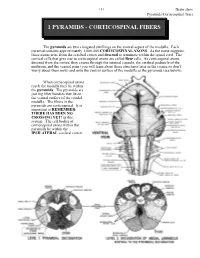
Corticospinal Fibers
151 Brain stem Pyramids/Corticospinal Tract 1 PYRAMIDS - CORTICOSPINAL FIBERS The pyramids are two elongated swellings on the ventral aspect of the medulla. Each pyramid contains approximately 1,000,000 CORTICOSPINAL AXONS. As the name suggests, these axons arise from the cerebral cortex and descend to terminate within the spinal cord. The cortical cells that give rise to corticospinal axons are called Betz cells. As corticospinal axons descend from the cortex, they course through the internal capsule, the cerebral peduncle of the midbrain, and the ventral pons (you will learn about these structures later in the course so don’t worry about them now) and onto the ventral surface of the medulla as the pyramids (see below). When corticospinal axons reach the medulla they lie within the pyramids. The pyramids are just big fiber bundles that lie on the ventral surface of the caudal medulla. The fibers in the pyramids are corticospinal. It is important to REMEMBER: THERE HAS BEEN NO CROSSING YET! in this system. The cell bodies of corticospinal axons within the pyramids lie within the IPSILATERAL cerebral cortex. Brain stem 152 Pyramids/Corticospinal Tract At the most caudal pole of the pyramids the corticospinal axons cross over the midline and now continue their descent on the contralateral (to the cell of origin) side. This crossover point is called the PYRAMIDAL DECUSSATION. The crossing fibers enter the lateral funiculus of the spinal cord where they are called the LATERAL CORTICOSPINAL TRACT (“corticospinal” is not good enough, you have to call them lateral corticospinal; LCST - remember this one??). LCST axons exit the tract to terminate upon neurons in the spinal cord gray matter along its entire length. -

The Superior and Inferior Colliculi of the Mole (Scalopus Aquaticus Machxinus)
THE SUPERIOR AND INFERIOR COLLICULI OF THE MOLE (SCALOPUS AQUATICUS MACHXINUS) THOMAS N. JOHNSON' Laboratory of Comparative Neurology, Departmmt of Amtomy, Un&versity of hfiehigan, Ann Arbor INTRODUCTION This investigation is a study of the afferent and efferent connections of the tectum of the midbrain in the mole (Scalo- pus aquaticus machrinus). An attempt is made to correlate these findings with the known habits of the animal. A subterranean animal of the middle western portion of the United States, Scalopus aquaticus machrinus is the largest of the genus Scalopus and its habits have been more thor- oughly studied than those of others of this genus according to Jackson ('15) and Hamilton ('43). This animal prefers a well-drained, loose soil. It usually frequents open fields and pastures but also is found in thin woods and meadows. Following a rain, new superficial burrows just below the surface of the ground are pushed in all directions to facili- tate the capture of worms and other soil life. Ten inches or more below the surface the regular permanent highway is constructed; the mole retreats here during long periods of dry weather or when frost is in the ground. The principal food is earthworms although, under some circumstances, larvae and adult insects are the more usual fare. It has been demonstrated conclusively that, under normal conditions, moles will eat vegetable matter. It seems not improbable that they may take considerable quantities of it at times. A dissertation submitted in partial fulfillment of the requirements for the degree of Doctor of Philosophy in the University of Michigan. -

Eye-Movement Studies of Visual Face Perception
Eye-movement studies of visual face perception Joseph Arizpe A dissertation submitted for the degree of Doctor of Philosophy of the University College London Institute of Cognitive Neuroscience University College London 2015 1 Declaration I, Joseph Arizpe, confirm that the work presented in this thesis is my own. Where information has been derived from other sources, I confirm that this has been indicated in the thesis. 2 Abstract This thesis investigates factors influencing eye-movement patterns during face perception, the relationship of eye-movement patterns to facial recognition performance, and methodological considerations impacting the detection of differences in eye-movement patterns. In particular, in the first study (chapter 2), in which the basis of the other-race effect was investigated, differences in eye- movement patterns during recognition of own- versus other-race (African, Chinese) faces were found for Caucasian participants. However, these eye- movement differences were subtle and analysis-dependent, indicating that the discrepancy in prior reports regarding the presence or absence of such differences are due to variability in statistical sensitivity of analysis methods across studies. The second and third studies (chapters 3 and 4) characterized visuomotor factors, specifically pre-stimulus start position and distance, which strongly influence subsequent eye-movement patterns during face perception. An overall bias in fixation patterns to the opposite side of the face induced by start position and an increasing undershoot of the first ordinal fixation with increasing start distance were found. These visuomotor influences were not specific to faces and did not depend on the predictability of the location of the upcoming stimulus. -

Direct Projections from Cochlear Nuclear Complex to Auditory Thalamus in the Rat
The Journal of Neuroscience, December 15, 2002, 22(24):10891–10897 Direct Projections from Cochlear Nuclear Complex to Auditory Thalamus in the Rat Manuel S. Malmierca,1 Miguel A. Mercha´n,1 Craig K. Henkel,2 and Douglas L. Oliver3 1Laboratory for the Neurobiology of Hearing, Institute for Neuroscience of Castilla y Leo´ n and Faculty of Medicine, University of Salamanca, 37007 Salamanca, Spain, 2Wake Forest University School of Medicine, Department of Neurobiology and Anatomy, Winston-Salem, North Carolina 27157-1010, and 3University of Connecticut Health Center, Department of Neuroscience, Farmington, Connecticut 06030-3401 It is known that the dorsal cochlear nucleus and medial genic- inferior colliculus and are widely distributed within the medial ulate body in the auditory system receive significant inputs from division of the medial geniculate, suggesting that the projection somatosensory and visual–motor sources, but the purpose of is not topographic. As a nonlemniscal auditory pathway that such inputs is not totally understood. Moreover, a direct con- parallels the conventional auditory lemniscal pathway, its func- nection of these structures has not been demonstrated, be- tions may be distinct from the perception of sound. Because cause it is generally accepted that the inferior colliculus is an this pathway links the parts of the auditory system with prom- obligatory relay for all ascending input. In the present study, we inent nonauditory, multimodal inputs, it may form a neural have used auditory neurophysiology, double labeling with an- network through which nonauditory sensory and visual–motor terograde tracers, and retrograde tracers to investigate the systems may modulate auditory information processing. -

Imaging of the Confused Patient: Toxic Metabolic Disorders Dara G
Imaging of the Confused Patient: Toxic Metabolic Disorders Dara G. Jamieson, M.D. Weill Cornell Medicine, New York, NY The patient who presents with either acute or subacute confusion, in the absence of a clearly defined speech disorder and focality on neurological examination that would indicate an underlying mass lesion, needs to be evaluated for a multitude of neurological conditions. Many of the conditions that produce the recent onset of alteration in mental status, that ranges from mild confusion to florid delirium, may be due to infectious or inflammatory conditions that warrant acute intervention such as antimicrobial drugs, steroids or plasma exchange. However, some patients with recent onset of confusion have an underlying toxic-metabolic disorders indicating a specific diagnosis with need for appropriate treatment. The clinical presentations of some patients may indicate the diagnosis (e.g. hypoglycemia, chronic alcoholism) while the imaging patterns must be recognized to make the diagnosis in other patients. Toxic-metabolic disorders constitute a group of diseases and syndromes with diverse causes and clinical presentations. Many toxic-metabolic disorders have no specific neuroimaging correlates, either at early clinical stages or when florid symptoms develop. However, some toxic-metabolic disorders have characteristic abnormalities on neuroimaging, as certain areas of the central nervous system appear particularly vulnerable to specific toxins and metabolic perturbations. Areas of particular vulnerability in the brain include: 1) areas of high-oxygen demand (e.g. basal ganglia, cerebellum, hippocampus), 2) the cerebral white matter and 3) the mid-brain. Brain areas of high-oxygen demand are particularly vulnerable to toxins that interfere with cellular respiratory metabolism. -

Efficacy And/Or Effectiveness of Portable Neuromodulation Stimulator (Pons®) As Treatment for Traumatic Brain Injury (TBI)”
Evidence-Based Practice Group Answers to Clinical Questions “Efficacy and/or Effectiveness of Portable Neuromodulation Stimulator (PoNS®) as Treatment for Traumatic Brain Injury (TBI)” A Rapid Systematic Review By WorkSafeBC Evidence-Based Practice Group Dr. Craig Martin Manager, Clinical Services Chair, Evidence-Based Practice Group October 2019 Clinical Services – Worker and Employer Services Efficacy and/or Effectiveness of Portable Neuromodulation Stimulator (PoNS®) as Treatment for Traumatic Brain Injury (TBI) i About this report Efficacy and/or Effectiveness of Portable Neuromodulation Stimulator (PoNS®) as Treatment for Traumatic Brain Injury (TBI) Published: October 2019 About the Evidence-Based Practice Group The Evidence-Based Practice Group was established to address the many medical and policy issues that WorkSafeBC officers deal with on a regular basis. Members apply established techniques of critical appraisal and evidence-based review of topics solicited from both WorkSafeBC staff and other interested parties such as surgeons, medical specialists, and rehabilitation providers. Suggested Citation WorkSafeBC Evidence-Based Practice Group, Martin CW. Efficacy and/or Effectiveness of Portable Neuromodulation Stimulator (PoNS®) as Treatment for Traumatic Brain Injury (TBI). Richmond, BC: WorksafeBC Evidence- Based Practice Group; October 2019. Contact Information Evidence-Based Practice Group WorkSafeBC PO Box 5350 Stn Terminal Vancouver BC V6B 5L5 Email [email protected] Phone 604 279-7417 Toll-free 1 888 967-5377 -

Eye Fields in the Frontal Lobes of Primates
Brain Research Reviews 32Ž. 2000 413±448 www.elsevier.comrlocaterbres Full-length review Eye fields in the frontal lobes of primates Edward J. Tehovnik ), Marc A. Sommer, I-Han Chou, Warren M. Slocum, Peter H. Schiller Department of Brain and CognitiÕe Sciences, Massachusetts Institute of Technology, E25-634, Cambridge, MA 02139, USA Accepted 19 October 1999 Abstract Two eye fields have been identified in the frontal lobes of primates: one is situated dorsomedially within the frontal cortex and will be referred to as the eye field within the dorsomedial frontal cortexŽ. DMFC ; the other resides dorsolaterally within the frontal cortex and is commonly referred to as the frontal eye fieldŽ. FEF . This review documents the similarities and differences between these eye fields. Although the DMFC and FEF are both active during the execution of saccadic and smooth pursuit eye movements, the FEF is more dedicated to these functions. Lesions of DMFC minimally affect the production of most types of saccadic eye movements and have no effect on the execution of smooth pursuit eye movements. In contrast, lesions of the FEF produce deficits in generating saccades to briefly presented targets, in the production of saccades to two or more sequentially presented targets, in the selection of simultaneously presented targets, and in the execution of smooth pursuit eye movements. For the most part, these deficits are prevalent in both monkeys and humans. Single-unit recording experiments have shown that the DMFC contains neurons that mediate both limb and eye movements, whereas the FEF seems to be involved in the execution of eye movements only. -

Visualization of the Pyramidal Decussation Utilizing Diffusion
OPEN ACCESS RESEARCH Visualization of the pyramidal decussation utilizing diffusion tensor imaging: a feasibility study utilizing generalized q-sampling imaging Erik H Middlebrooks1,*, Jeffrey A Bennett1, Sharatchandra Bidari1, and Alissa Old Crow1 1Department of Radiology, University of Florida College of Medicine, Gainesville, Florida, USA *corresponding author ([email protected]) Abstract Background: The delineating point of the end of the brainstem and beginning of the spinal cord is known as the cervicomedullary junction (CMJ). This point is defined by the decussation of the pyramidal tracts. Abnormal configuration and location of the CMJ have both been implicated in disease processes such as Chiari malformation. Unfortunately, the CMJ is not directly visualized on contemporary imaging techniques. Diffusion tensor imaging (DTI) has given us the ability to directly visualize white matter tracts, but suffers from difficulties with visualizing crossing fibers. Many advanced techniques for visualizing crossing fibers utilize substantially long imaging times or non-clinical magnet strengths making clinical applicability limited. Objective: This study inves- tigates the use of generalized q-sampling imaging with diffusion decomposition on standard DTI acquisitions at 3 Tesla to demonstrate the pyramidal decussation. Methods: Three differing DTI protocols were analyzed in vivo with scan times of 17 minutes, 10 minutes, and 5.5 minutes. Data was processed with standard DTI post-processing, as well as generalized q-sampling imaging with diffusion decomposition. Results: The results of the study show that the pyramidal decussation can be reliably visualized using generalized q-sampling imaging and diffusion decomposition with scan times as low at 10 minutes. Conclusion: Utilizing generalized q-sampling post-processing, the pyramidal decussation can be reliably visualized using clinically feasible DTI sequences with scan times as low as 10 minutes. -

Auditory and Vestibular Systems Objective • to Learn the Functional
Auditory and Vestibular Systems Objective • To learn the functional organization of the auditory and vestibular systems • To understand how one can use changes in auditory function following injury to localize the site of a lesion • To begin to learn the vestibular pathways, as a prelude to studying motor pathways controlling balance in a later lab. Ch 7 Key Figs: 7-1; 7-2; 7-4; 7-5 Clinical Case #2 Hearing loss and dizziness; CC4-1 Self evaluation • Be able to identify all structures listed in key terms and describe briefly their principal functions • Use neuroanatomy on the web to test your understanding ************************************************************************************** List of media F-5 Vestibular efferent connections The first order neurons of the vestibular system are bipolar cells whose cell bodies are located in the vestibular ganglion in the internal ear (NTA Fig. 7-3). The distal processes of these cells contact the receptor hair cells located within the ampulae of the semicircular canals and the utricle and saccule. The central processes of the bipolar cells constitute the vestibular portion of the vestibulocochlear (VIIIth cranial) nerve. Most of these primary vestibular afferents enter the ipsilateral brain stem inferior to the inferior cerebellar peduncle to terminate in the vestibular nuclear complex, which is located in the medulla and caudal pons. The vestibular nuclear complex (NTA Figs, 7-2, 7-3), which lies in the floor of the fourth ventricle, contains four nuclei: 1) the superior vestibular nucleus; 2) the inferior vestibular nucleus; 3) the lateral vestibular nucleus; and 4) the medial vestibular nucleus. Vestibular nuclei give rise to secondary fibers that project to the cerebellum, certain motor cranial nerve nuclei, the reticular formation, all spinal levels, and the thalamus. -

A Small Dorsal Pontine Infarction Presenting with Total Gaze Palsy Including Vertical Saccades and Pursuit
Journal of Clinical Neurology / Volume 3 / December, 2007 Case Report A Small Dorsal Pontine Infarction Presenting with Total Gaze Palsy Including Vertical Saccades and Pursuit Eugene Lee, M.D., Ji Soo Kim, M.D.a, Jong Sung Kim, M.D., Ph.D., Ha Seob Song, M.D., Seung Min Kim, M.D., Sun Uk Kwon, M.D. Department of Neurology, Asan Medical Center, University of Ulsan College of Medicine aDepartment of Neurology, Seoul National University, Bundang Hospital A small localized infarction in the dorsal pontine area can cause various eye-movement disturbances, such as abducens palsy, horizontal conjugate gaze palsy, internuclear ophthalmoplegia, and one-and-a-half syndrome. However, complete loss of vertical saccades and pursuit with horizontal gaze palsy has not been reported previously in a patient with a small pontine lesion. We report a 67-year-old man with a small dorsal caudal pontine infarct who exhibited total horizontal gaze palsy as well as loss of vertical saccades and pursuit. J Clin Neurol 3(4):208-211, 2007 Key Words : Ophthalmoplegia, Pontine infarction, Omnipause neurons A small localized dorsal pontine infarction can to admission he had experienced sudden general produce abducens palsy, horizontal conjugate gaze weakness for approximately 20 minutes without loss palsy, internuclear ophthalmoplegia (INO), and one- of consciousness while working on his farm. The and-a-half syndrome by damaging the abducens nucleus following day, the patient experienced dysarthric and its fascicle, the paramedian pontine reticular speech and visual obscuration, and his family members formation (PPRF), or the medial longitudinal fasciculus noticed that his eyes were deviated to one side.