APS Abstracts Submitted for Presentation at the 2002 APS
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Phytopythium: Molecular Phylogeny and Systematics
Persoonia 34, 2015: 25–39 www.ingentaconnect.com/content/nhn/pimj RESEARCH ARTICLE http://dx.doi.org/10.3767/003158515X685382 Phytopythium: molecular phylogeny and systematics A.W.A.M. de Cock1, A.M. Lodhi2, T.L. Rintoul 3, K. Bala 3, G.P. Robideau3, Z. Gloria Abad4, M.D. Coffey 5, S. Shahzad 6, C.A. Lévesque 3 Key words Abstract The genus Phytopythium (Peronosporales) has been described, but a complete circumscription has not yet been presented. In the present paper we provide molecular-based evidence that members of Pythium COI clade K as described by Lévesque & de Cock (2004) belong to Phytopythium. Maximum likelihood and Bayesian LSU phylogenetic analysis of the nuclear ribosomal DNA (LSU and SSU) and mitochondrial DNA cytochrome oxidase Oomycetes subunit 1 (COI) as well as statistical analyses of pairwise distances strongly support the status of Phytopythium as Oomycota a separate phylogenetic entity. Phytopythium is morphologically intermediate between the genera Phytophthora Peronosporales and Pythium. It is unique in having papillate, internally proliferating sporangia and cylindrical or lobate antheridia. Phytopythium The formal transfer of clade K species to Phytopythium and a comparison with morphologically similar species of Pythiales the genera Pythium and Phytophthora is presented. A new species is described, Phytopythium mirpurense. SSU Article info Received: 28 January 2014; Accepted: 27 September 2014; Published: 30 October 2014. INTRODUCTION establish which species belong to clade K and to make new taxonomic combinations for these species. To achieve this The genus Pythium as defined by Pringsheim in 1858 was goal, phylogenies based on nuclear LSU rRNA (28S), SSU divided by Lévesque & de Cock (2004) into 11 clades based rRNA (18S) and mitochondrial DNA cytochrome oxidase1 (COI) on molecular systematic analyses. -

Characterization of the Ergosterol Biosynthesis Pathway in Ceratocystidaceae
Journal of Fungi Article Characterization of the Ergosterol Biosynthesis Pathway in Ceratocystidaceae Mohammad Sayari 1,2,*, Magrieta A. van der Nest 1,3, Emma T. Steenkamp 1, Saleh Rahimlou 4 , Almuth Hammerbacher 1 and Brenda D. Wingfield 1 1 Department of Biochemistry, Genetics and Microbiology, Forestry and Agricultural Biotechnology Institute (FABI), University of Pretoria, Pretoria 0002, South Africa; [email protected] (M.A.v.d.N.); [email protected] (E.T.S.); [email protected] (A.H.); brenda.wingfi[email protected] (B.D.W.) 2 Department of Plant Science, University of Manitoba, 222 Agriculture Building, Winnipeg, MB R3T 2N2, Canada 3 Biotechnology Platform, Agricultural Research Council (ARC), Onderstepoort Campus, Pretoria 0110, South Africa 4 Department of Mycology and Microbiology, University of Tartu, 14A Ravila, 50411 Tartu, Estonia; [email protected] * Correspondence: [email protected]; Fax: +1-204-474-7528 Abstract: Terpenes represent the biggest group of natural compounds on earth. This large class of organic hydrocarbons is distributed among all cellular organisms, including fungi. The different classes of terpenes produced by fungi are mono, sesqui, di- and triterpenes, although triterpene ergosterol is the main sterol identified in cell membranes of these organisms. The availability of genomic data from members in the Ceratocystidaceae enabled the detection and characterization of the genes encoding the enzymes in the mevalonate and ergosterol biosynthetic pathways. Using Citation: Sayari, M.; van der Nest, a bioinformatics approach, fungal orthologs of sterol biosynthesis genes in nine different species M.A.; Steenkamp, E.T.; Rahimlou, S.; of the Ceratocystidaceae were identified. -

Pythium Root Rot to Always Act the Same
pests & diseases DON’T EXPECT PYTHIUM ROOT ROT TO ALWAYS ACT THE SAME Cornell University trials are teaching researchers more about this troublesome pathogen, how it interacts with the plants it infects and how it is becoming more difficult to control — and what they’ve learned may surprise you. Seedling geraniums inoculated with Pythium may be stunted or killed. By Gary W. Moorman (Photos courtesy of Margery Daughtrey) and Margery L. Daughtrey ne new development impor- tems because it has a swimming spore stage. pasteurization could lead to Pythium outbreaks. tant to our understanding of Pythium irregulare also forms swimming spores Second, Pythium ultimum favors cool greenhouse Pythium species comes from and is isolated from a very wide variety of temperatures: the minimum for growth is 41° F, the findings of molecular greenhouse crops, almost any crop grown. It is maximum 95° F and optimum 77-86° F. When geneticists. We have long less aggressive than P. aphanidermatum, often other organisms are inhibited by cool tempera- thoughtO of Pythium as a “water mold fun- causing stunting but seldom killing plants quick- ture, P. ultimum can prosper. gus”— now it has been reclassified according to ly. Pythium ultimum, very commonly noted in P. aphanidermatum has a higher minimum tem- information gained from comparing gene simi- old clinic records, is much less common but is perature (50° F) than P. ultimum and a very high larities…and Pythium is no longer a fungus! still isolated from chrysanthemums, verbenas, optimum temperature at 95-104° F. This Pythium DNA analysis has shown us that Pythium is geraniums and sometimes poinsettias. -

Research Collection
Research Collection Doctoral Thesis Studies on Venturiaceae on Rosaceous plants Author(s): Menon, Radha Publication Date: 1956 Permanent Link: https://doi.org/10.3929/ethz-a-000092066 Rights / License: In Copyright - Non-Commercial Use Permitted This page was generated automatically upon download from the ETH Zurich Research Collection. For more information please consult the Terms of use. ETH Library Diss ETH Prom. Nr. 2585 B Studies on Venturiaceae on Rosaceous Plants THESIS PRESENTED TO THE SWISS FEDERAL INSTITUTE OF TECHNOLOGY ZURICH FOR THE DEGREE OF DOCTOR OF NATURAL SCIENCES BY RADHA MENON at CITIZEN OF Ser\ INDIA Accepted on the recommendation of Prof. Dr. E. Gaumann and Prof. Dr. A. Frey-Wyssling 19 5 6 Druck von A. W. Hayn's Erben, Berlin SO 36 Veroffentlicht in „Phytopathologische Zcitschrift" Band 27, Heft 2, Seite 117 bis 146 (1956) Verlag Paul Parey, Berlin und Hamburg From the Department of special Botany of the Swiss Federal Institute of Technology in Zurich Director: Prof. Dr. E. Gdumann Studies on Venturiaceae on Rosaceous Plants By Radha Menon With 10 Figures Contents: I. General Introduction. A. Venturiaceae. B. Venturiaceae on Rosaceae: 1) Venturia, 2) Coleroa, 3) Gibbera, 4) Xenomeris, 5) Apiosporina. — II. Experimental Part. A. Cultural Studies. B. Inoculation Experiments: 1) Introduction, 2) Inoculation Studies, 3) Results, 4) Conclusions. — III. Morphological and Cultural Studies. A. Genus Venturia: 1) Venturia inaequalis, 2) Venturia tomentosae, 3) Venturia pirina, 4) Venturia pruni-cerasi, 5) Venturia Mullcri, 6) Venturia potentillae, 7) Venturia palustris, 8) Venturia alchemillae. — Appendix: Fusicladium eriobotryae. — B. Genus Coleroa: Coleroa chac- tomium. — C. Genus Gibbera: Gibbera rosae. -

(US) 38E.85. a 38E SEE", A
USOO957398OB2 (12) United States Patent (10) Patent No.: US 9,573,980 B2 Thompson et al. (45) Date of Patent: Feb. 21, 2017 (54) FUSION PROTEINS AND METHODS FOR 7.919,678 B2 4/2011 Mironov STIMULATING PLANT GROWTH, 88: R: g: Ei. al. 1 PROTECTING PLANTS FROM PATHOGENS, 3:42: ... g3 is et al. A61K 39.00 AND MMOBILIZING BACILLUS SPORES 2003/0228679 A1 12.2003 Smith et al." ON PLANT ROOTS 2004/OO77090 A1 4/2004 Short 2010/0205690 A1 8/2010 Blä sing et al. (71) Applicant: Spogen Biotech Inc., Columbia, MO 2010/0233.124 Al 9, 2010 Stewart et al. (US) 38E.85. A 38E SEE",teWart et aal. (72) Inventors: Brian Thompson, Columbia, MO (US); 5,3542011/0321197 AllA. '55.12/2011 SE",Schön et al.i. Katie Thompson, Columbia, MO (US) 2012fO259101 A1 10, 2012 Tan et al. 2012fO266327 A1 10, 2012 Sanz Molinero et al. (73) Assignee: Spogen Biotech Inc., Columbia, MO 2014/0259225 A1 9, 2014 Frank et al. US (US) FOREIGN PATENT DOCUMENTS (*) Notice: Subject to any disclaimer, the term of this CA 2146822 A1 10, 1995 patent is extended or adjusted under 35 EP O 792 363 B1 12/2003 U.S.C. 154(b) by 0 days. EP 1590466 B1 9, 2010 EP 2069504 B1 6, 2015 (21) Appl. No.: 14/213,525 WO O2/OO232 A2 1/2002 WO O306684.6 A1 8, 2003 1-1. WO 2005/028654 A1 3/2005 (22) Filed: Mar. 14, 2014 WO 2006/O12366 A2 2/2006 O O WO 2007/078127 A1 7/2007 (65) Prior Publication Data WO 2007/086898 A2 8, 2007 WO 2009037329 A2 3, 2009 US 2014/0274707 A1 Sep. -
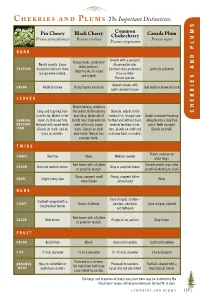
C P the Important Distinctions
C P The Important Distinctions S Common M Pin Cherry Black Cherry Chokecherry Canada Plum Prunus pennsylvanica Prunus serotina Prunus nigra U Prunus virginiana L P BARK D Smooth with a pungent, Young trunks: prominent N Nearly smooth. Large disagreeable odor. white lenticals. TEXTURE horizontal lenticels show Lenticels less prominent Lenticels yellowish A Older trunks: fissured orange when rubbed. than on other and ridged. Prunus species. S E Grayish-brown, with I COLOR Reddish-brown Young trunks are black Dull reddish-brown to black light-colored fissures R LEAVES R E Elliptic/oblong, widest in H Long and tapering from the center, thick leathery Obovate, widest in the C base to tip. Widest in the and shiny. Underside of terminal 1⁄3, sharply saw- Ovate or obovate tapering GENERAL lower 1⁄3; thin and firm midrib near stalk end cov- toothed and without hairs, abruptly into a long thin DESCRIP- textured with round teeth. ered with rusty, brown medium leathery in tex- point. Teeth rounded. TION Glands on stalk, and no hairs. Glands on stalk ture, glands on stalk and Glands on stalk. hairs on midribs. near blade. Margin has no brown hairs on midrib. rounded teeth. TWIGS Thorns common on SHAPE Very fine Waxy Medium slender older twigs Red-brown with a lighter Current growth gray, older COLOR Red and reddish-brown Gray or purplish-brown or greenish margin growth darkening to black Sharp, pungent smell Strong, pungent bitter- ODOR Slight cherry odor None when broken almond odor BUDS Cone shaped, slender – Football-shaped with a SHAPE Ovate, -

Rhynchosporium Secalis (Oud.) Davis and Barley Leaf Scald in South Australia
*^'lii i;['tìrulrr LIßI{ÅIìY Rlrynchosporíum secølis (Oud.) Davis and Barleyl-eaf Scafd in SouthAustralia J.^4. Davidson Depar"lrnent of Plant Science Thesis submitted for Master of Agricultural Science, University of Adelaide, MaY, L992 Pæp AIMS I LITERATIIRE REVIEW 1ll CHAPTER 1: SURVEY OF THE PATHOGENICITY RANGE OF RITVNCHOSPORIUM SECALIS, THE CAUSAI PATHOGEN OF BARLEY LEAF SCALD, IN SOUTH AUSTRALIA. INTRODUCTION 2 GENERAI MATERIALS 2 A. MOBILE NURSERIES Materials and Methods 4 Results 6 B. GLASSHOUSE TESTING Materials and Methods 16 Results 18 C. DISCUSSION % CHAPTER 2: MEASUREMENT OF RESISTANCE TO RHYNCHOSPORIUM SECALIS IN BARLEY, IN THE FIELD AND THE GLASSHOUSE. INTRODUCTION 38 A. FIELD SCREENING Materials and Methods 39 Results 42 B. GLASSHOUSE SCREENING Materials and Methods 46 l) CoupARrsoN oF SpRAy INocur,¿.uoN AND SINGLE DROPLET INOCULATION (i) Spray Inoculation Materials and Methods 47 Results ß (ii) Single Droplet Inoculation Materials and Methods 53 Results 53 Discussion 57 B) MEASUREMENT OF DISEASE COMPONENTS (i) Comparison of four Rhynchosporiurn secalis pathotypes 1 Materials and Methods 58 {,t Results 60 (ii) Effect of inoculum concentration Materials and Methods 72 Results 73 (iii) Measurement of disease components on sixteen barley lines Materials and Methods 80 Results 81 C. DISCUSSION m I CHAPTER 8: YIELD LOSSES IN BARLEY ASSOCIATED WITH LEAF SCALD 4 ,t,l it INTRODUCTION gI, Materials and Methods 95 Results 103 i L DISCUSSION TN 'tj 'l i CHAPTER 4: GENERAL DISCUSSION 7ß I APPEÎ{DICES II ji ,l 't itl i'l "l BIBLIOGRAPITY XXVII ll f,,{ ,] l ! I : I I fl Abstract Title: Rhynchosporium secalis (Oud.) Davis and Barley Leaf Scald in South Australia. -

Sand Plums for Home and Commercial Production
Oklahoma Cooperative Extension Service HLA-6258 Sand Plums for Home and Commercial Production Beth McMahon Oklahoma Cooperative Extension Fact Sheets Research Assistant Oklahoma State University are also available on our website at: http://osufacts.okstate.edu Bruce Dunn Assistant Professor Geyer, 2010). Flowering will last for a couple of weeks and Oklahoma State University either red or yellow fruit will begin to form afterward. Ripening of the fruit occurs from June to early August and are either Sand plums, also known as Chickasaw plum, Cherokee yellow or a bright red. Both colors occur in the same areas plum, or Sandhill plum (Prunus angustifolia Marshall), are native of Oklahoma. Fruit size can range from ¼ inch to 1 inch. It fruit-producing shrubs or small trees in Oklahoma (Figure 1). is recommended that long sleeves be worn while collecting Use of sand plums range from cover for native bird species fruit since the plants may be thorny, depending upon how to making jams, jellies, and wine from the fruit. Commercial damaged they have been by deer and cattle in the past. desire in making jams and jellies has led to a rising interest in cultivating sand plums for home and orchard production. The purpose of this publication is to provide some basic knowledge Selecting Plants on how to identify, propagate, and grow your own sand plums. Besides selecting plants for fruit size and crop load, Sand plums range from 2 feet to 25 feet high, depend- you may also want to consider selecting plants that have ing upon soil and water conditions (Row and Geyer, 2010). -

Co-Adaptations Between Ceratocystidaceae Ambrosia Fungi and the Mycangia of Their Associated Ambrosia Beetles
Iowa State University Capstones, Theses and Graduate Theses and Dissertations Dissertations 2018 Co-adaptations between Ceratocystidaceae ambrosia fungi and the mycangia of their associated ambrosia beetles Chase Gabriel Mayers Iowa State University Follow this and additional works at: https://lib.dr.iastate.edu/etd Part of the Biodiversity Commons, Biology Commons, Developmental Biology Commons, and the Evolution Commons Recommended Citation Mayers, Chase Gabriel, "Co-adaptations between Ceratocystidaceae ambrosia fungi and the mycangia of their associated ambrosia beetles" (2018). Graduate Theses and Dissertations. 16731. https://lib.dr.iastate.edu/etd/16731 This Dissertation is brought to you for free and open access by the Iowa State University Capstones, Theses and Dissertations at Iowa State University Digital Repository. It has been accepted for inclusion in Graduate Theses and Dissertations by an authorized administrator of Iowa State University Digital Repository. For more information, please contact [email protected]. Co-adaptations between Ceratocystidaceae ambrosia fungi and the mycangia of their associated ambrosia beetles by Chase Gabriel Mayers A dissertation submitted to the graduate faculty in partial fulfillment of the requirements for the degree of DOCTOR OF PHILOSOPHY Major: Microbiology Program of Study Committee: Thomas C. Harrington, Major Professor Mark L. Gleason Larry J. Halverson Dennis V. Lavrov John D. Nason The student author, whose presentation of the scholarship herein was approved by the program of study committee, is solely responsible for the content of this dissertation. The Graduate College will ensure this dissertation is globally accessible and will not permit alterations after a degree is conferred. Iowa State University Ames, Iowa 2018 Copyright © Chase Gabriel Mayers, 2018. -

Trichoderma: the “Secrets” of a Multitalented Biocontrol Agent
plants Review Trichoderma: The “Secrets” of a Multitalented Biocontrol Agent 1, 1, 2 3 Monika Sood y, Dhriti Kapoor y, Vipul Kumar , Mohamed S. Sheteiwy , Muthusamy Ramakrishnan 4 , Marco Landi 5,6,* , Fabrizio Araniti 7 and Anket Sharma 4,* 1 School of Bioengineering and Biosciences, Lovely Professional University, Jalandhar-Delhi G.T. Road (NH-1), Phagwara, Punjab 144411, India; [email protected] (M.S.); [email protected] (D.K.) 2 School of Agriculture, Lovely Professional University, Delhi-Jalandhar Highway, Phagwara, Punjab 144411, India; [email protected] 3 Department of Agronomy, Faculty of Agriculture, Mansoura University, Mansoura 35516, Egypt; [email protected] 4 State Key Laboratory of Subtropical Silviculture, Zhejiang A&F University, Hangzhou 311300, China; [email protected] 5 Department of Agriculture, University of Pisa, I-56124 Pisa, Italy 6 CIRSEC, Centre for Climatic Change Impact, University of Pisa, Via del Borghetto 80, I-56124 Pisa, Italy 7 Dipartimento AGRARIA, Università Mediterranea di Reggio Calabria, Località Feo di Vito, SNC I-89124 Reggio Calabria, Italy; [email protected] * Correspondence: [email protected] (M.L.); [email protected] (A.S.) Authors contributed equal. y Received: 25 May 2020; Accepted: 16 June 2020; Published: 18 June 2020 Abstract: The plant-Trichoderma-pathogen triangle is a complicated web of numerous processes. Trichoderma spp. are avirulent opportunistic plant symbionts. In addition to being successful plant symbiotic organisms, Trichoderma spp. also behave as a low cost, effective and ecofriendly biocontrol agent. They can set themselves up in various patho-systems, have minimal impact on the soil equilibrium and do not impair useful organisms that contribute to the control of pathogens. -

Kentucky Fruit Facts
Kentucky Fruit Facts January-February Newsletter 2018 http://www.uky.edu/hort/documents-list-fruit-facts Cooperative Extension Service John Strang, Extension Fruit Specialist, Editor University of Kentucky Horticulture Department Denise Stephens, Newsletter Designer 1100 So. Limestone St. Lexington Ky 40546-0091 (859) 257-2909 Inside this Issue: Fax: (859) 257-2859 Fruit Crop News . .1 extension.ca.uky.edu Upcoming Meetings. .2 Thornless Erect Blackberry Cultivar Trial . 3 of the state showing that we had a few spots that Pesticide Certification: Who Needs It? . .4 were particularly cold and peach flower bud damage Black Knot. .6 probably occurred in these areas. The temperatures Cane Diseases of Brambles. .6 below 0°F more than likely caused damage to Receiving Fruit Facts on the Internet . .8 thornless blackberries. The rule of thumb is that for every degree below 0°F thornless blackberries lose 10% of the crop. Keep in mind that these Fruit Crop News low temperatures occurred in early January when John Strang, U.K. Extension Horticulturist and Matt Dixon, blackberries were at their maximum hardiness level U.K. Ag Meteorologist so there may not be any injury to plants where the temperature was a few degrees below 0°F. We are hopefully through the coldest portion While pruning watch for signs of San Jose of the winter and it is time to start pruning apple and Scale in your trees. Feeding by each insect causes pear trees. Prune the oldest trees first leaving your an increase in the reddish purple pigments beneath youngest until later in the spring. If you have semi- the bark leaving a small circular spot around the dwarf or dwarf trees that are spaced out and trained to feeding site. -

Novel Species of Huntiella from Naturally-Occurring Forest Trees in Greece and South Africa
A peer-reviewed open-access journal MycoKeys 69: 33–52 (2020) Huntiella species in Greece and South Africa 33 doi: 10.3897/mycokeys.69.53205 RESEARCH ARTICLE MycoKeys http://mycokeys.pensoft.net Launched to accelerate biodiversity research Novel species of Huntiella from naturally-occurring forest trees in Greece and South Africa FeiFei Liu1,2, Seonju Marincowitz1, ShuaiFei Chen1,2, Michael Mbenoun1, Panaghiotis Tsopelas3, Nikoleta Soulioti3, Michael J. Wingfield1 1 Department of Biochemistry, Genetics and Microbiology (BGM), Forestry and Agricultural Biotechnology In- stitute (FABI), University of Pretoria, Pretoria 0028, South Africa 2 China Eucalypt Research Centre (CERC), Chinese Academy of Forestry (CAF), ZhanJiang, 524022, GuangDong Province, China 3 Institute of Mediter- ranean Forest Ecosystems, Terma Alkmanos, 11528 Athens, Greece Corresponding author: ShuaiFei Chen ([email protected]) Academic editor: R. Phookamsak | Received 13 April 2020 | Accepted 4 June 2020 | Published 10 July 2020 Citation: Liu FF, Marincowitz S, Chen SF, Mbenoun M, Tsopelas P, Soulioti N, Wingfield MJ (2020) Novel species of Huntiella from naturally-occurring forest trees in Greece and South Africa. MycoKeys 69: 33–52. https://doi. org/10.3897/mycokeys.69.53205 Abstract Huntiella species are wood-infecting, filamentous ascomycetes that occur in fresh wounds on a wide va- riety of tree species. These fungi are mainly known as saprobes although some have been associated with disease symptoms. Six fungal isolates with typical culture characteristics of Huntiella spp. were collected from wounds on native forest trees in Greece and South Africa. The aim of this study was to identify these isolates, using morphological characters and multigene phylogenies of the rRNA internal transcribed spacer (ITS) region, portions of the β-tubulin (BT1) and translation elongation factor 1α (TEF-1α) genes.