Bioactivation of N-Alkyl Substituted Phosphor- Amidothioate Insecticides
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Chemical Name Federal P Code CAS Registry Number Acutely
Acutely / Extremely Hazardous Waste List Federal P CAS Registry Acutely / Extremely Chemical Name Code Number Hazardous 4,7-Methano-1H-indene, 1,4,5,6,7,8,8-heptachloro-3a,4,7,7a-tetrahydro- P059 76-44-8 Acutely Hazardous 6,9-Methano-2,4,3-benzodioxathiepin, 6,7,8,9,10,10- hexachloro-1,5,5a,6,9,9a-hexahydro-, 3-oxide P050 115-29-7 Acutely Hazardous Methanimidamide, N,N-dimethyl-N'-[2-methyl-4-[[(methylamino)carbonyl]oxy]phenyl]- P197 17702-57-7 Acutely Hazardous 1-(o-Chlorophenyl)thiourea P026 5344-82-1 Acutely Hazardous 1-(o-Chlorophenyl)thiourea 5344-82-1 Extremely Hazardous 1,1,1-Trichloro-2, -bis(p-methoxyphenyl)ethane Extremely Hazardous 1,1a,2,2,3,3a,4,5,5,5a,5b,6-Dodecachlorooctahydro-1,3,4-metheno-1H-cyclobuta (cd) pentalene, Dechlorane Extremely Hazardous 1,1a,3,3a,4,5,5,5a,5b,6-Decachloro--octahydro-1,2,4-metheno-2H-cyclobuta (cd) pentalen-2- one, chlorecone Extremely Hazardous 1,1-Dimethylhydrazine 57-14-7 Extremely Hazardous 1,2,3,4,10,10-Hexachloro-6,7-epoxy-1,4,4,4a,5,6,7,8,8a-octahydro-1,4-endo-endo-5,8- dimethanonaph-thalene Extremely Hazardous 1,2,3-Propanetriol, trinitrate P081 55-63-0 Acutely Hazardous 1,2,3-Propanetriol, trinitrate 55-63-0 Extremely Hazardous 1,2,4,5,6,7,8,8-Octachloro-4,7-methano-3a,4,7,7a-tetra- hydro- indane Extremely Hazardous 1,2-Benzenediol, 4-[1-hydroxy-2-(methylamino)ethyl]- 51-43-4 Extremely Hazardous 1,2-Benzenediol, 4-[1-hydroxy-2-(methylamino)ethyl]-, P042 51-43-4 Acutely Hazardous 1,2-Dibromo-3-chloropropane 96-12-8 Extremely Hazardous 1,2-Propylenimine P067 75-55-8 Acutely Hazardous 1,2-Propylenimine 75-55-8 Extremely Hazardous 1,3,4,5,6,7,8,8-Octachloro-1,3,3a,4,7,7a-hexahydro-4,7-methanoisobenzofuran Extremely Hazardous 1,3-Dithiolane-2-carboxaldehyde, 2,4-dimethyl-, O- [(methylamino)-carbonyl]oxime 26419-73-8 Extremely Hazardous 1,3-Dithiolane-2-carboxaldehyde, 2,4-dimethyl-, O- [(methylamino)-carbonyl]oxime. -

Acutely / Extremely Hazardous Waste List
Acutely / Extremely Hazardous Waste List Federal P CAS Registry Acutely / Extremely Chemical Name Code Number Hazardous 4,7-Methano-1H-indene, 1,4,5,6,7,8,8-heptachloro-3a,4,7,7a-tetrahydro- P059 76-44-8 Acutely Hazardous 6,9-Methano-2,4,3-benzodioxathiepin, 6,7,8,9,10,10- hexachloro-1,5,5a,6,9,9a-hexahydro-, 3-oxide P050 115-29-7 Acutely Hazardous Methanimidamide, N,N-dimethyl-N'-[2-methyl-4-[[(methylamino)carbonyl]oxy]phenyl]- P197 17702-57-7 Acutely Hazardous 1-(o-Chlorophenyl)thiourea P026 5344-82-1 Acutely Hazardous 1-(o-Chlorophenyl)thiourea 5344-82-1 Extemely Hazardous 1,1,1-Trichloro-2, -bis(p-methoxyphenyl)ethane Extemely Hazardous 1,1a,2,2,3,3a,4,5,5,5a,5b,6-Dodecachlorooctahydro-1,3,4-metheno-1H-cyclobuta (cd) pentalene, Dechlorane Extemely Hazardous 1,1a,3,3a,4,5,5,5a,5b,6-Decachloro--octahydro-1,2,4-metheno-2H-cyclobuta (cd) pentalen-2- one, chlorecone Extemely Hazardous 1,1-Dimethylhydrazine 57-14-7 Extemely Hazardous 1,2,3,4,10,10-Hexachloro-6,7-epoxy-1,4,4,4a,5,6,7,8,8a-octahydro-1,4-endo-endo-5,8- dimethanonaph-thalene Extemely Hazardous 1,2,3-Propanetriol, trinitrate P081 55-63-0 Acutely Hazardous 1,2,3-Propanetriol, trinitrate 55-63-0 Extemely Hazardous 1,2,4,5,6,7,8,8-Octachloro-4,7-methano-3a,4,7,7a-tetra- hydro- indane Extemely Hazardous 1,2-Benzenediol, 4-[1-hydroxy-2-(methylamino)ethyl]- 51-43-4 Extemely Hazardous 1,2-Benzenediol, 4-[1-hydroxy-2-(methylamino)ethyl]-, P042 51-43-4 Acutely Hazardous 1,2-Dibromo-3-chloropropane 96-12-8 Extemely Hazardous 1,2-Propylenimine P067 75-55-8 Acutely Hazardous 1,2-Propylenimine 75-55-8 Extemely Hazardous 1,3,4,5,6,7,8,8-Octachloro-1,3,3a,4,7,7a-hexahydro-4,7-methanoisobenzofuran Extemely Hazardous 1,3-Dithiolane-2-carboxaldehyde, 2,4-dimethyl-, O- [(methylamino)-carbonyl]oxime 26419-73-8 Extemely Hazardous 1,3-Dithiolane-2-carboxaldehyde, 2,4-dimethyl-, O- [(methylamino)-carbonyl]oxime. -

Environmental Health Criteria 63 ORGANOPHOSPHORUS
Environmental Health Criteria 63 ORGANOPHOSPHORUS INSECTICIDES: A GENERAL INTRODUCTION Please note that the layout and pagination of this web version are not identical with the printed version. Organophophorus insecticides: a general introduction (EHC 63, 1986) INTERNATIONAL PROGRAMME ON CHEMICAL SAFETY ENVIRONMENTAL HEALTH CRITERIA 63 ORGANOPHOSPHORUS INSECTICIDES: A GENERAL INTRODUCTION This report contains the collective views of an international group of experts and does not necessarily represent the decisions or the stated policy of the United Nations Environment Programme, the International Labour Organisation, or the World Health Organization. Published under the joint sponsorship of the United Nations Environment Programme, the International Labour Organisation, and the World Health Organization World Health Orgnization Geneva, 1986 The International Programme on Chemical Safety (IPCS) is a joint venture of the United Nations Environment Programme, the International Labour Organisation, and the World Health Organization. The main objective of the IPCS is to carry out and disseminate evaluations of the effects of chemicals on human health and the quality of the environment. Supporting activities include the development of epidemiological, experimental laboratory, and risk-assessment methods that could produce internationally comparable results, and the development of manpower in the field of toxicology. Other activities carried out by the IPCS include the development of know-how for coping with chemical accidents, coordination -

Appendix Common, Trade, and Chemical Names of Pesticides Mentioned in the Present Volume
Appendix Common, trade, and chemical names of pesticides mentioned in the present volume Commonname Tradename Chemical name aldrin Octalene 1,2,3,4,10,1 O-hexachloro-1 ,4,4a,S,8,8a hexahydro-1,4-endo-exo-S,8-dimethano naphthalene amidithion Thiocron O,O-dimethyl S-(2-methoxyethyl carbamoylmethyl) phosphorodithioate azinphos methyl Guthion, Gusathion O,O-dimcthyl S-( 4-oxo-l ,2,3-benzotri azin-3-( 4H)-ylmethyl) phosphorodithioate captan Orthocid N -(trichloromethylthio) cyclohex-4-ene- 1,2-dicarboximide carbaryl Sevin 1-naphthyl-mcthylcarbamate carbophenothion Trithion O,O-diethyl S-[(p-chlorophenylthio) methyl] phosphorodithioate chlorfenvinphos Birlane (Shell), 2-chloro-1-(2,4-dichlorophenyl) vinyl Sapecron (e/BA) diethyl phosphate chlorphenamidine Galecron N'-( 4-chloro-o-tolyl)-N,N-dimethyl formamidine chlorthion Chlorthion O,O-dimethyIO-(3-chloro-4-nitro phenyl)-phosphorothioate coumaphos Asuntol, Co-Ral O,O-diethyl O-(3-chloro-4-methyl- 2-oxo-2H-1-benzopyran-7-yl) phosphorothioate DDT Gesarol 1,1,1-trichloro-2,2-bis(p-chlorophenyl) ethane demeton Systox O,O-diethyl O-(and S)-2-(ethylthio)ethyl phosphorothioates DEF S,S,S-tributyltrithiophosphate demeton methyl Metasystox O,O-dimethyl O-(and S)-2-(ethylthio) ethyl phosphorothioates diazinon Diazinon, Basudin O,O-diethyIO-(2-isopropyl-4-methyl- 6-pyrimidyl) phosphorothioate dichlorvos Vapona (Shell), O,O-dimethyl-2,2-dichlorovinyl Nuvan (e/BA) phosphate dicrotophos Bidrin (Shell), O,O-dimethyl O-(2-dimethyl-carbamyl-1- Carbicron (e/BA) methyl) vinyl phosphate dieldrin Octalox -

NMP-Free Formulations of Neonicotinoids
(19) & (11) EP 2 266 400 A1 (12) EUROPEAN PATENT APPLICATION (43) Date of publication: (51) Int Cl.: 29.12.2010 Bulletin 2010/52 A01N 43/40 (2006.01) A01N 43/86 (2006.01) A01N 47/40 (2006.01) A01N 51/00 (2006.01) (2006.01) (2006.01) (21) Application number: 09305544.0 A01P 7/00 A01N 25/02 (22) Date of filing: 15.06.2009 (84) Designated Contracting States: (72) Inventors: AT BE BG CH CY CZ DE DK EE ES FI FR GB GR • Gasse, Jean-Jacques HR HU IE IS IT LI LT LU LV MC MK MT NL NO PL 27600 Saint-Aubin-Sur-Gaillon (FR) PT RO SE SI SK TR • Duchamp, Guillaume Designated Extension States: 92230 Gennevilliers (FR) AL BA RS • Cantero, Maria 92230 Gennevilliers (FR) (71) Applicant: NUFARM 92233 Gennevelliers (FR) (74) Representative: Cabinet Plasseraud 52, rue de la Victoire 75440 Paris Cedex 09 (FR) (54) NMP-free formulations of neonicotinoids (57) The invention relates to NMP-free liquid formulation comprising at least one nicotinoid and at least one aprotic polar component selected from the group comprising the compounds of formula I, II or III below, and mixtures thereof, wherein R1 and R2 independently represent H or an alkyl group having less than 5 carbons, preferably a methyl group, and n represents an integer ranging from 0 to 5, and to their applications. EP 2 266 400 A1 Printed by Jouve, 75001 PARIS (FR) EP 2 266 400 A1 Description Technical Field of the invention 5 [0001] The invention relates to novel liquid formulations of neonicotinoids and to their use for treating plants, for protecting plants from pests and/or for controlling pests infestation. -
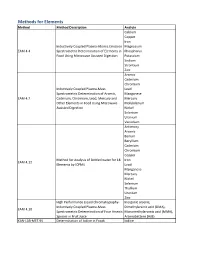
Method Description
Methods for Elements Method Method Description Analyte Calcium Copper Iron Inductively Coupled Plasma-Atomic Emission Magnesium EAM 4.4 Spectrometric Determination of Elements in Phosphorus Food Using Microwave Assisted Digestion Potassium Sodium Strontium Zinc Arsenic Cadmium Chromium Inductively Coupled Plasma-Mass Lead Spectrometric Determination of Arsenic, Manganese EAM 4.7 Cadmium, Chromium, Lead, Mercury and Mercury Other Elements in Food Using Microwave Molybdenum Assisted Digestion Nickel Selenium Uranium Vanadium Antimony Arsenic Barium Beryllium Cadmium Chromium Copper Method for Analysis of Bottled water for 18 Iron EAM 4.12 Elements by ICPMS Lead Manganese Mercury Nickel Selenium Thallium Uranium Zinc High Performance Liquid Chromatography- Inorganic arsenic, Inductively Coupled Plasma-Mass Dimethylarsinic acid (DMA), EAM 4.10 Spectrometric Determination of Four Arsenic Monomethylarsonic acid (MMA), Species in Fruit Juice Arsenobetaine (AsB) KAN-LAB-MET.95 Determination of Iodine in Foods Iodine Methods for Radionuclides Method Method Description Analyte Determination of Strontium-90 in Foods by WEAC.RN.METHOD.2.0 Strontium-90 Internal Gas-Flow Proportional Counting Americium-241 Cesium-134 Cesium-137 Determination of Gamma-Ray Emitting Cobalt-60 WEAC.RN.METHOD.3.0 Radionuclides in Foods by High-Purity Potassium-40 Germanium Spectrometry Radium-226 Ruthenium-103 Ruthenium-106 Thorium-232 Methods for Pesticides/Industrial Chemicals Method Method Description Analyte Extraction Method: Analysis of Pesticides KAN-LAB-PES.53 and -

The Hazardous Substances and Waste Dangerous Goods Regulations
1 HAZARDOUS SUBSTANCES AND WASTE DANGEROUS GOODS E-10.2 REG 3 The Hazardous Substances and Waste Dangerous Goods Regulations being Chapter E-10.2 Reg 3 (effective April 1, 1989) as amended by Saskatchewan Regulations 25/92, 107/92, 28/94, 3/95 and 63/2000. NOTE: This consolidation is not official. Amendments have been incorporated for convenience of reference and the original statutes and regulations should be consulted for all purposes of interpretation and application of the law. In order to preserve the integrity of the original statutes and regulations, errors that may have appeared are reproduced in this consolidation. 2 HAZARDOUS SUBSTANCES AND E-10.2 REG 3 WASTE DANGEROUS GOODS Table of Contents 1 Title 11 Decision to grant approval INTERPRETATION 12 Approval not assignable, exception 2 Interpretation 13 Duties of operator, owner HAZARDOUS SUBSTANCES 14 Prohibition re storage in above-ground tanks 3 Designation of hazardous substances 15 Prohibition re storage in underground tanks 16 Prohibition re storage in certain containers or DESIGNATION AS HAZARDOUS WASTES stockpiles 3.1 Designation of waste dangerous goods 17 Decommissioning as hazardous wastes TRANSFERAL OF WASTE DANGEROUS GOODS CHARACTERIZATION OF SUBSTANCES 18 Transferal of waste dangerous goods 4 Characteristics of certain hazardous substances EXEMPTION FROM REQUIREMENTS Appendix A 5 General Exemptions INDUSTRIAL HAZARDOUS SUBSTANCES 6 Underground storage facilities Appendix B 7 Above-ground storage facilities ACUTE HAZARDOUS SUBSTANCES 8 Storage in small containers Appendix C APPROVAL TO STORE ENVIRONMENTAL PERSISTENT OR 9 Approval to store CHRONIC HAZARDOUS SUBSTANCES APPROVAL TO CONSTRUCT Appendix D 10 Approval to construct WASTE DANGEROUS GOODS 3 HAZARDOUS SUBSTANCES AND WASTE DANGEROUS GOODS E-10.2 REG 3 CHAPTER E-10.2 REG 3 The Environmental Management and Protection Act Title 1 These regulations may be cited as The Hazardous Substances and Waste Dangerous Goods Regulations. -

Comparing Pesticide Regulations After NAFTA
University of Arkansas System Division of Agriculture [email protected] | (479) 575-7646 An Agricultural Law Research Article U.S. and Mexican Regulation of Methyl Bromide: Comparing Pesticide Regulations After NAFTA by Kyle W. Lathrop and Cindy K. Bushur-Hallam Originally published in OKLAHOMA LAW REVIEW 48 OKLA. L. REV. 289 (1995) www.NationalAgLawCenter.org U.S. AND MEXICAN REGULATION OF METHYL BROMIDE: COMPARING PESTICIDE REGULATIONS AFTER NAFTA KYLE W. LATHROP* & CINDY K. BUSHUR-HALLAM** I. Introduction Pesticides are one of this century's great agricultural ironies. Pesticide use allows for tremendous increases in productivity and permits food and fiber production in areas that would have been impractical to cultivate without pesticides.' Conversely. pesticides prove to be one of the significant human environmental dilemmas of this century: persistence and toxicity of pesticides. misuse in applications. and pest resistance all extract a toll on human health and the environment A necessary role for pesticides exists, but policy makers must account for potential costs and balance them against the benefits to ensure that pesticide benefits do not come at the expense of high health and environmental costs. This need, to balance costs against benefits, is intensified in the realm of agricultural trade, as pesticides and products grown with pesticides move across political boundaries. Part of this problem stems from differing pesticide regulations for production. use, and residue tolerances in different countrles,2 but the lack of economic rationales in creating policies compounds the problem. A need to combine the law • Temporary Instructor, University ofGeorgia, College ofAgricultural and Environmental Sciences, Athens, Georgia; LL.M. -

United States Patent (19) 11 3,941,829 Pissiotas Et Al
United States Patent (19) 11 3,941,829 Pissiotas et al. (45) Mar. 2, 1976 54 N-PHENYL-N'-CARBOPHENOXY FORMAMIDINES 57 ABSTRACT (75) Inventors: Georg Pissiotas, Lorrach, Germany; Phenylformamidines of the formula Dieter Dürr, Bottmingen, Switzerland 4 R3 (73) Assignee: Ciba-Geigy Corporation, Ardsley, R N.Y. R5 NiccH-N / (22 Filed: Dec. 1, 1972 N (21) Appl. No.: 311,058 COOR2 R6 R7 30 Foreign Application Priority Data Dec. 7, 1971 Switzerland....................... 17790/7 wherein R represents hydrogen, alkyl, alkenyl or al Dec. 7, 1971 Switzerland....................... 1779/7 kynyl, R represents a-naphthyl, Jan. 26, 1972 Switzerland......................... 1224/72 Oct. 27, 1972 Switzerland....................... 5729/72 52) U.S. Cl...... 260f471 C; 260/240 G; 260/465 D; 260/470; 260/472; 424/277; 424/278; 424/285; 424/300 (51) int. Cl......................................... C07C 125/06 (58) Field of Search..... 260/471 C, 472, 470, 240 G 56 References Cited or substituted phenyl, FOREIGN PATENTS OR APPLICATIONS wherein the phenyl group is not substituted simulta 890,922 lf 972 Canada neously in the 2-position by a methyl group and in the 2,202,034 | 972 Germany 4-position by a chlorine atom, R. R. R. R. and R. 2,123,001 81972 France represent one or more radicals which are the same or 778,383 7/1972 Belgium different, such as hydrogen or halogen atoms or alkyl, alkoxy, alkylthio, alkenyloxy, alkynyloxy, alkoxycar Primary Examiner-Anton H. Sutto bonyl, CFs, cyano or nitro groups, their process for Assistant Examiner-Michael Shippen the manufacture and their use in pest control. -

Saimiri Sciureus) David Lee Hopper Iowa State University
Iowa State University Capstones, Theses and Retrospective Theses and Dissertations Dissertations 1976 Delayed matching to sample performance and parathion toxicity in the squirrel monkey (Saimiri sciureus) David Lee Hopper Iowa State University Follow this and additional works at: https://lib.dr.iastate.edu/rtd Part of the Experimental Analysis of Behavior Commons, and the Psychiatry and Psychology Commons Recommended Citation Hopper, David Lee, "Delayed matching to sample performance and parathion toxicity in the squirrel monkey (Saimiri sciureus) " (1976). Retrospective Theses and Dissertations. 5746. https://lib.dr.iastate.edu/rtd/5746 This Dissertation is brought to you for free and open access by the Iowa State University Capstones, Theses and Dissertations at Iowa State University Digital Repository. It has been accepted for inclusion in Retrospective Theses and Dissertations by an authorized administrator of Iowa State University Digital Repository. For more information, please contact [email protected]. INFORMATION TO USERS This material was produced from a microfilm copy of the original document. While the most advanced technological means to photograph and reproduce this document have been used, the quality is heavily dependent upon the quality of the original submitted. The following explanation of techniques is provided to help you understand markings or patterns which may appear on this reproduction. 1. The sign or "target" for pages apparently lacking from the document photographed is ' Missing Page(s)". If it was possible to obtain the missing page(s) or section, they are spliced into the film along with adjacent pages. This may have necessitated cutting thru an image and duplicating adjacent pages to insure you complete continuity. -

Pesticides and You News from Beyond Pesticides: Protecting Health and the Environment with Science, Policy & Action Volume 35, Number 4 Winter 2015-16
Pesticides and You News from Beyond Pesticides: Protecting Health and the Environment with Science, Policy & Action Volume 35, Number 4 Winter 2015-16 Cultivating Plants that Poison A Special Report on Systemic Pesticides Also in this issue: What’s Up With Organic Standards? Letter from Washington 35 Years of Progress and the Tipping Point Within Our Sight eyond Pesticides in 2016 celebrates a rich 35-year history of agricultural systems can feed the world better than chemical-intensive accomplishments in organic policy and land management. We approaches. While we tinkered with integrated pest management Bare honored to be part of an extensive network of communities, (IPM) as an agricultural tool to reduce pesticide dependency, the lack people, organizations, scientists, and practitioners that educates on of a holistic approach to soil health, protection of biodiversity, and the hazards of pesticides, while moving decision and policy makers the identification of least-toxic inputs diminished its effectiveness as a to adopt sustainable organic practices. With its proven viability, we long-term sustainability approach in land management. see within our sights the opportunity for a societal transformation to organic practices that protect the environment on which life depends. I served on the National Organic Standards Board (NOSB) for a 5-year term (2010-2015). Having spearheaded the 1982 Organic Farming Meeting the urgent challenges Act, which became the 1983 Agricultural Productivity Act and USDA’s We use this occasion only to reenergize ourselves to meet the urgent Low-Input Sustainable Agriculture (LISA) program, and then the 1990 challenges ahead. We are at the center of the organic transformation Organic Foods Production Act (OFPA), we knew we had tremendous that crosses issues of clean air and water, healthy food, and soil practices institutional barriers within USDA to implement policies that defied that build organic matter, sequesters carbon, and slows climate change. -

Array Biosensor for the Detection of Organophosphates
ARRAY BIOSENSOR FOR THE DETECTION OF ORGANOPHOSPHATES Except where reference is made to the work of others, the work described in this thesis is my own or was done in collaboration with my advisory committee. This thesis does not include any proprietary or classified information __________________________________________ Madhumati Ramanathan Certificate of Approval: _________________________ __________________________ Bart C. Prorok Aleksandr L. Simonian, Chair Assistant Professor Associate Professor Materials Engineering Materials Engineering _________________________ __________________________ Jeffrey W. Fergus Zhong Y. Cheng Associate Professor Assistant Professor Materials Engineering Materials Engineering ___________________________ Stephen L. McFarland Acting Dean Graduate School ARRAY BIOSENSOR FOR THE DETECTION OF ORGANOPHOSPHATES Madhumati Ramanathan A Thesis Submitted to the Graduate Faculty of Auburn University in Partial Fulfillment of the Requirements for the Degree of Master of Science Auburn, Alabama August 7, 2006 ARRAY BIOSENSOR FOR THE DETECTION OF ORGANOPHOSPHATES Madhumati Ramanathan Permission is granted to Auburn University to make copies of this thesis at its discretion, upon the request of individuals or institutions at their expense. The author reserves all publication rights. _____________________________ Signature of Author _____________________________ Date of graduation iii VITA Madhumati Ramanathan, daughter of S. Ramanathan and R. Sakunthalai, was born on May 23, 1982 in Chennai, TN India. She earned the degree of Bachelor of Engineering in Metallurgical Engineering from Government College of Engineering, Periyar University, Salem, India in 2003. iv THESIS ABSTRACT ARRAY BIOSENSOR FOR THE DETECTION OF ORGANOPHOSPHATES Madhumati Ramanathan Master of Science, August 7, 2006 (B.S., Periyar University, 2003) 113 Typed Pages Directed by Aleksandr L. Simonian The aim of the current study was to develop an optical-based array biosensor for enzyme kinetics monitoring by fluorescence spectroscopy.