Our Investigations on Saligenin Cyclic Phos Phorus Esters Have Started
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Ortho-Cresyl-Phosphate Poisoning
J Neurol Neurosurg Psychiatry: first published as 10.1136/jnnp.25.3.234 on 1 August 1962. Downloaded from J. Neurol. Neurosurg. Psychiat., 1962, 25, 234 Toxic polyneuritis in Bombay due to ortho-cresyl-phosphate poisoning D. D. VORA, DARAB K. DASTUR, BEATRIZ M. BRAGANCA, L. M. PARIHAR, C. G. S. IYER, R. B. FONDEKAR, AND K. PRABHAKARAN From Lokmanya Tilak Municipal General Hospital, Sion, Neurology Unit, Indian Council of Medical Research, and Department of Enzyme Chemistry, Indian Cancer Research Centre, Bombay Since 1930 poisoning with ortho-cresyl-phosphate purchased food from the same grocer in their neigh- has been recognized as a cause of peripheral poly- bourhood. He also mentioned that others staying in neuritis (Smith, Elvove, and Frazier, 1930). Ortho- the same locality and consuming mustard oil, but cresyl-phosphate (O.C.P.) is widely used as a purchasing their food from other grocers, were not plasticiser and in the production of heat-stable affected. The examination of the other affected per- lubricating oils. Many cases of industrial poisoning sons revealed identical histories and clinical pictures. with O.C.P. have been reported since then but as a In this group there was no history of gastro-enteritis result of strict precautionary measures it has now or of febrile illness preceding the paralysis. These become a rarity. However, the occasional contamina- patients also asserted that all those who were buying is not uncommon. their food from the same grocer but who were not tion of food with O.C.P. Protected by copyright. Poisoning with O.C.P. -

Chemical Name Federal P Code CAS Registry Number Acutely
Acutely / Extremely Hazardous Waste List Federal P CAS Registry Acutely / Extremely Chemical Name Code Number Hazardous 4,7-Methano-1H-indene, 1,4,5,6,7,8,8-heptachloro-3a,4,7,7a-tetrahydro- P059 76-44-8 Acutely Hazardous 6,9-Methano-2,4,3-benzodioxathiepin, 6,7,8,9,10,10- hexachloro-1,5,5a,6,9,9a-hexahydro-, 3-oxide P050 115-29-7 Acutely Hazardous Methanimidamide, N,N-dimethyl-N'-[2-methyl-4-[[(methylamino)carbonyl]oxy]phenyl]- P197 17702-57-7 Acutely Hazardous 1-(o-Chlorophenyl)thiourea P026 5344-82-1 Acutely Hazardous 1-(o-Chlorophenyl)thiourea 5344-82-1 Extremely Hazardous 1,1,1-Trichloro-2, -bis(p-methoxyphenyl)ethane Extremely Hazardous 1,1a,2,2,3,3a,4,5,5,5a,5b,6-Dodecachlorooctahydro-1,3,4-metheno-1H-cyclobuta (cd) pentalene, Dechlorane Extremely Hazardous 1,1a,3,3a,4,5,5,5a,5b,6-Decachloro--octahydro-1,2,4-metheno-2H-cyclobuta (cd) pentalen-2- one, chlorecone Extremely Hazardous 1,1-Dimethylhydrazine 57-14-7 Extremely Hazardous 1,2,3,4,10,10-Hexachloro-6,7-epoxy-1,4,4,4a,5,6,7,8,8a-octahydro-1,4-endo-endo-5,8- dimethanonaph-thalene Extremely Hazardous 1,2,3-Propanetriol, trinitrate P081 55-63-0 Acutely Hazardous 1,2,3-Propanetriol, trinitrate 55-63-0 Extremely Hazardous 1,2,4,5,6,7,8,8-Octachloro-4,7-methano-3a,4,7,7a-tetra- hydro- indane Extremely Hazardous 1,2-Benzenediol, 4-[1-hydroxy-2-(methylamino)ethyl]- 51-43-4 Extremely Hazardous 1,2-Benzenediol, 4-[1-hydroxy-2-(methylamino)ethyl]-, P042 51-43-4 Acutely Hazardous 1,2-Dibromo-3-chloropropane 96-12-8 Extremely Hazardous 1,2-Propylenimine P067 75-55-8 Acutely Hazardous 1,2-Propylenimine 75-55-8 Extremely Hazardous 1,3,4,5,6,7,8,8-Octachloro-1,3,3a,4,7,7a-hexahydro-4,7-methanoisobenzofuran Extremely Hazardous 1,3-Dithiolane-2-carboxaldehyde, 2,4-dimethyl-, O- [(methylamino)-carbonyl]oxime 26419-73-8 Extremely Hazardous 1,3-Dithiolane-2-carboxaldehyde, 2,4-dimethyl-, O- [(methylamino)-carbonyl]oxime. -

The Effects of Occupational Exposure to Chlorpyrifos on the Peripheral
201 Occup Environ Med: first published as 10.1136/oem.2003.008847 on 25 February 2004. Downloaded from ORIGINAL ARTICLE The effects of occupational exposure to chlorpyrifos on the peripheral nervous system: a prospective cohort study J W Albers, D H Garabrant, S J Schweitzer, R P Garrison, R J Richardson, S Berent ............................................................................................................................... Occup Environ Med 2004;61:201–211. doi: 10.1136/oem.2003.008847 Aims: To determine whether chronic occupational exposure to chlorpyrifos at levels associated with various aspects of manufacturing produced a clinically evident or subclinical peripheral neuropathy. Methods: Clinical and quantitative nerve conduction study (NCS) examinations were performed on two occasions on chlorpyrifos manufacturing workers who had measurable chlorpyrifos exposure and a referent group. Baseline evaluations were performed on 53 of 66 eligible chlorpyrifos subjects and on 60 of 74 eligible referent subjects; one-year evaluations were completed on 111 of the 113 subjects evaluated at baseline. Results: Chlorpyrifos and referent groups differed significantly in measures of 3,5,6 trichloro-2-pyridinol excretion and plasma butyrylcholinesterase (BuChE) activity, indicating substantially higher exposures See end of article for authors’ affiliations among chlorpyrifos subjects. Few subjects had clinically important neurological symptoms or signs. NCS ....................... results were comparable to control values, and there were no significant group differences in NCS results at baseline, one year, or change over one year. No chlorpyrifos subject fulfilled conventional criteria for Correspondence to: Dr J W Albers, Department confirmed peripheral neuropathy at baseline or one-year examinations. The odds ratios for developing of Neurology, 1C325/ any diagnosable level of peripheral neuropathy among the chlorpyrifos subjects was not increased at 0032 University Hospital, baseline or at one year compared to referents at baseline. -

Enzymatic Degradation of Organophosphorus Pesticides and Nerve Agents by EC: 3.1.8.2
catalysts Review Enzymatic Degradation of Organophosphorus Pesticides and Nerve Agents by EC: 3.1.8.2 Marek Matula 1, Tomas Kucera 1 , Ondrej Soukup 1,2 and Jaroslav Pejchal 1,* 1 Department of Toxicology and Military Pharmacy, Faculty of Military Health Sciences, University of Defence, Trebesska 1575, 500 01 Hradec Kralove, Czech Republic; [email protected] (M.M.); [email protected] (T.K.); [email protected] (O.S.) 2 Biomedical Research Center, University Hospital Hradec Kralove, Sokolovska 581, 500 05 Hradec Kralove, Czech Republic * Correspondence: [email protected] Received: 26 October 2020; Accepted: 20 November 2020; Published: 24 November 2020 Abstract: The organophosphorus substances, including pesticides and nerve agents (NAs), represent highly toxic compounds. Standard decontamination procedures place a heavy burden on the environment. Given their continued utilization or existence, considerable efforts are being made to develop environmentally friendly methods of decontamination and medical countermeasures against their intoxication. Enzymes can offer both environmental and medical applications. One of the most promising enzymes cleaving organophosphorus compounds is the enzyme with enzyme commission number (EC): 3.1.8.2, called diisopropyl fluorophosphatase (DFPase) or organophosphorus acid anhydrolase from Loligo Vulgaris or Alteromonas sp. JD6.5, respectively. Structure, mechanisms of action and substrate profiles are described for both enzymes. Wild-type (WT) enzymes have a catalytic activity against organophosphorus compounds, including G-type nerve agents. Their stereochemical preference aims their activity towards less toxic enantiomers of the chiral phosphorus center found in most chemical warfare agents. Site-direct mutagenesis has systematically improved the active site of the enzyme. These efforts have resulted in the improvement of catalytic activity and have led to the identification of variants that are more effective at detoxifying both G-type and V-type nerve agents. -

Acutely / Extremely Hazardous Waste List
Acutely / Extremely Hazardous Waste List Federal P CAS Registry Acutely / Extremely Chemical Name Code Number Hazardous 4,7-Methano-1H-indene, 1,4,5,6,7,8,8-heptachloro-3a,4,7,7a-tetrahydro- P059 76-44-8 Acutely Hazardous 6,9-Methano-2,4,3-benzodioxathiepin, 6,7,8,9,10,10- hexachloro-1,5,5a,6,9,9a-hexahydro-, 3-oxide P050 115-29-7 Acutely Hazardous Methanimidamide, N,N-dimethyl-N'-[2-methyl-4-[[(methylamino)carbonyl]oxy]phenyl]- P197 17702-57-7 Acutely Hazardous 1-(o-Chlorophenyl)thiourea P026 5344-82-1 Acutely Hazardous 1-(o-Chlorophenyl)thiourea 5344-82-1 Extemely Hazardous 1,1,1-Trichloro-2, -bis(p-methoxyphenyl)ethane Extemely Hazardous 1,1a,2,2,3,3a,4,5,5,5a,5b,6-Dodecachlorooctahydro-1,3,4-metheno-1H-cyclobuta (cd) pentalene, Dechlorane Extemely Hazardous 1,1a,3,3a,4,5,5,5a,5b,6-Decachloro--octahydro-1,2,4-metheno-2H-cyclobuta (cd) pentalen-2- one, chlorecone Extemely Hazardous 1,1-Dimethylhydrazine 57-14-7 Extemely Hazardous 1,2,3,4,10,10-Hexachloro-6,7-epoxy-1,4,4,4a,5,6,7,8,8a-octahydro-1,4-endo-endo-5,8- dimethanonaph-thalene Extemely Hazardous 1,2,3-Propanetriol, trinitrate P081 55-63-0 Acutely Hazardous 1,2,3-Propanetriol, trinitrate 55-63-0 Extemely Hazardous 1,2,4,5,6,7,8,8-Octachloro-4,7-methano-3a,4,7,7a-tetra- hydro- indane Extemely Hazardous 1,2-Benzenediol, 4-[1-hydroxy-2-(methylamino)ethyl]- 51-43-4 Extemely Hazardous 1,2-Benzenediol, 4-[1-hydroxy-2-(methylamino)ethyl]-, P042 51-43-4 Acutely Hazardous 1,2-Dibromo-3-chloropropane 96-12-8 Extemely Hazardous 1,2-Propylenimine P067 75-55-8 Acutely Hazardous 1,2-Propylenimine 75-55-8 Extemely Hazardous 1,3,4,5,6,7,8,8-Octachloro-1,3,3a,4,7,7a-hexahydro-4,7-methanoisobenzofuran Extemely Hazardous 1,3-Dithiolane-2-carboxaldehyde, 2,4-dimethyl-, O- [(methylamino)-carbonyl]oxime 26419-73-8 Extemely Hazardous 1,3-Dithiolane-2-carboxaldehyde, 2,4-dimethyl-, O- [(methylamino)-carbonyl]oxime. -

Bioactivation of N-Alkyl Substituted Phosphor- Amidothioate Insecticides
J. Pesticide Sci. 9, 675-680 (1984) Original Article Bioactivation of N-Alkyl Substituted Phosphor- amidothioate Insecticides Masako UEJI and Chojiro ToMIzAWA National Institute of Agro-Environmental Sciences, Yatabe, Tsukuba-gun, Ibaraki 305, Japan (Received May 15, 1984) The insecticidal activity of O-ethyl O-2-isopropoxycarbonylphenyl N-alkylphoshor- amidothioates was examined with reference to their activation in biologicalsystems. Toxicity to the adzuki bean weevil varied with different N-alkyl groups. N-isopropylphosphoramido- thioate was the most toxic of the compounds tested, and N-unsubstituted, N-methyl- and N-ethylphosphoramidothioates were more toxic than fenitrothion. However, N-propyl and N-butyl homologs were less active than fenitrothion with the exception of the N-isopropyl homolog. LD50 values of the phosphoramidothioates for the insect were not correlated with in vitro anti-AChE activity of the phosphoramidates. 150 of N-isopropylphosphoramidate for acetylcholinesterases from adzuki bean weevil and bovine serum was higher than 10-3 M. When the phosphoramidothioates and phosphoramidate were incubated with rat liver micro- somal system, AChE activity of bovine serum was strongly inhibited in the presence of NADPH. Inhibition of AChE activity was reduced by addition of SKF 525-A to the micro- somal system. The compounds became also potent inhibitors for AChE by treatment with m-chloroperbenzoic acid. From these results, it was concluded that phosphoramidothioates and their oxons were activated oxidatively to inhibit AChE by the microsomal system as well as chemical treatment with peracid. of amide groups of phosphoramidothioate or INTRODUCTION phosphoramidate insecticides except schradan Bioactivity of phosphoramidothioates is vari- (octamethylpyrophosphoramidate). Moreover, able with structural changes. -

Environmental Health Criteria 63 ORGANOPHOSPHORUS
Environmental Health Criteria 63 ORGANOPHOSPHORUS INSECTICIDES: A GENERAL INTRODUCTION Please note that the layout and pagination of this web version are not identical with the printed version. Organophophorus insecticides: a general introduction (EHC 63, 1986) INTERNATIONAL PROGRAMME ON CHEMICAL SAFETY ENVIRONMENTAL HEALTH CRITERIA 63 ORGANOPHOSPHORUS INSECTICIDES: A GENERAL INTRODUCTION This report contains the collective views of an international group of experts and does not necessarily represent the decisions or the stated policy of the United Nations Environment Programme, the International Labour Organisation, or the World Health Organization. Published under the joint sponsorship of the United Nations Environment Programme, the International Labour Organisation, and the World Health Organization World Health Orgnization Geneva, 1986 The International Programme on Chemical Safety (IPCS) is a joint venture of the United Nations Environment Programme, the International Labour Organisation, and the World Health Organization. The main objective of the IPCS is to carry out and disseminate evaluations of the effects of chemicals on human health and the quality of the environment. Supporting activities include the development of epidemiological, experimental laboratory, and risk-assessment methods that could produce internationally comparable results, and the development of manpower in the field of toxicology. Other activities carried out by the IPCS include the development of know-how for coping with chemical accidents, coordination -

Recommended Classification of Pesticides by Hazard and Guidelines to Classification 2019 Theinternational Programme on Chemical Safety (IPCS) Was Established in 1980
The WHO Recommended Classi cation of Pesticides by Hazard and Guidelines to Classi cation 2019 cation Hazard of Pesticides by and Guidelines to Classi The WHO Recommended Classi The WHO Recommended Classi cation of Pesticides by Hazard and Guidelines to Classi cation 2019 The WHO Recommended Classification of Pesticides by Hazard and Guidelines to Classification 2019 TheInternational Programme on Chemical Safety (IPCS) was established in 1980. The overall objectives of the IPCS are to establish the scientific basis for assessment of the risk to human health and the environment from exposure to chemicals, through international peer review processes, as a prerequisite for the promotion of chemical safety, and to provide technical assistance in strengthening national capacities for the sound management of chemicals. This publication was developed in the IOMC context. The contents do not necessarily reflect the views or stated policies of individual IOMC Participating Organizations. The Inter-Organization Programme for the Sound Management of Chemicals (IOMC) was established in 1995 following recommendations made by the 1992 UN Conference on Environment and Development to strengthen cooperation and increase international coordination in the field of chemical safety. The Participating Organizations are: FAO, ILO, UNDP, UNEP, UNIDO, UNITAR, WHO, World Bank and OECD. The purpose of the IOMC is to promote coordination of the policies and activities pursued by the Participating Organizations, jointly or separately, to achieve the sound management of chemicals in relation to human health and the environment. WHO recommended classification of pesticides by hazard and guidelines to classification, 2019 edition ISBN 978-92-4-000566-2 (electronic version) ISBN 978-92-4-000567-9 (print version) ISSN 1684-1042 © World Health Organization 2020 Some rights reserved. -

Appendix Common, Trade, and Chemical Names of Pesticides Mentioned in the Present Volume
Appendix Common, trade, and chemical names of pesticides mentioned in the present volume Commonname Tradename Chemical name aldrin Octalene 1,2,3,4,10,1 O-hexachloro-1 ,4,4a,S,8,8a hexahydro-1,4-endo-exo-S,8-dimethano naphthalene amidithion Thiocron O,O-dimethyl S-(2-methoxyethyl carbamoylmethyl) phosphorodithioate azinphos methyl Guthion, Gusathion O,O-dimcthyl S-( 4-oxo-l ,2,3-benzotri azin-3-( 4H)-ylmethyl) phosphorodithioate captan Orthocid N -(trichloromethylthio) cyclohex-4-ene- 1,2-dicarboximide carbaryl Sevin 1-naphthyl-mcthylcarbamate carbophenothion Trithion O,O-diethyl S-[(p-chlorophenylthio) methyl] phosphorodithioate chlorfenvinphos Birlane (Shell), 2-chloro-1-(2,4-dichlorophenyl) vinyl Sapecron (e/BA) diethyl phosphate chlorphenamidine Galecron N'-( 4-chloro-o-tolyl)-N,N-dimethyl formamidine chlorthion Chlorthion O,O-dimethyIO-(3-chloro-4-nitro phenyl)-phosphorothioate coumaphos Asuntol, Co-Ral O,O-diethyl O-(3-chloro-4-methyl- 2-oxo-2H-1-benzopyran-7-yl) phosphorothioate DDT Gesarol 1,1,1-trichloro-2,2-bis(p-chlorophenyl) ethane demeton Systox O,O-diethyl O-(and S)-2-(ethylthio)ethyl phosphorothioates DEF S,S,S-tributyltrithiophosphate demeton methyl Metasystox O,O-dimethyl O-(and S)-2-(ethylthio) ethyl phosphorothioates diazinon Diazinon, Basudin O,O-diethyIO-(2-isopropyl-4-methyl- 6-pyrimidyl) phosphorothioate dichlorvos Vapona (Shell), O,O-dimethyl-2,2-dichlorovinyl Nuvan (e/BA) phosphate dicrotophos Bidrin (Shell), O,O-dimethyl O-(2-dimethyl-carbamyl-1- Carbicron (e/BA) methyl) vinyl phosphate dieldrin Octalox -

NMP-Free Formulations of Neonicotinoids
(19) & (11) EP 2 266 400 A1 (12) EUROPEAN PATENT APPLICATION (43) Date of publication: (51) Int Cl.: 29.12.2010 Bulletin 2010/52 A01N 43/40 (2006.01) A01N 43/86 (2006.01) A01N 47/40 (2006.01) A01N 51/00 (2006.01) (2006.01) (2006.01) (21) Application number: 09305544.0 A01P 7/00 A01N 25/02 (22) Date of filing: 15.06.2009 (84) Designated Contracting States: (72) Inventors: AT BE BG CH CY CZ DE DK EE ES FI FR GB GR • Gasse, Jean-Jacques HR HU IE IS IT LI LT LU LV MC MK MT NL NO PL 27600 Saint-Aubin-Sur-Gaillon (FR) PT RO SE SI SK TR • Duchamp, Guillaume Designated Extension States: 92230 Gennevilliers (FR) AL BA RS • Cantero, Maria 92230 Gennevilliers (FR) (71) Applicant: NUFARM 92233 Gennevelliers (FR) (74) Representative: Cabinet Plasseraud 52, rue de la Victoire 75440 Paris Cedex 09 (FR) (54) NMP-free formulations of neonicotinoids (57) The invention relates to NMP-free liquid formulation comprising at least one nicotinoid and at least one aprotic polar component selected from the group comprising the compounds of formula I, II or III below, and mixtures thereof, wherein R1 and R2 independently represent H or an alkyl group having less than 5 carbons, preferably a methyl group, and n represents an integer ranging from 0 to 5, and to their applications. EP 2 266 400 A1 Printed by Jouve, 75001 PARIS (FR) EP 2 266 400 A1 Description Technical Field of the invention 5 [0001] The invention relates to novel liquid formulations of neonicotinoids and to their use for treating plants, for protecting plants from pests and/or for controlling pests infestation. -
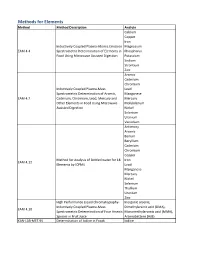
Method Description
Methods for Elements Method Method Description Analyte Calcium Copper Iron Inductively Coupled Plasma-Atomic Emission Magnesium EAM 4.4 Spectrometric Determination of Elements in Phosphorus Food Using Microwave Assisted Digestion Potassium Sodium Strontium Zinc Arsenic Cadmium Chromium Inductively Coupled Plasma-Mass Lead Spectrometric Determination of Arsenic, Manganese EAM 4.7 Cadmium, Chromium, Lead, Mercury and Mercury Other Elements in Food Using Microwave Molybdenum Assisted Digestion Nickel Selenium Uranium Vanadium Antimony Arsenic Barium Beryllium Cadmium Chromium Copper Method for Analysis of Bottled water for 18 Iron EAM 4.12 Elements by ICPMS Lead Manganese Mercury Nickel Selenium Thallium Uranium Zinc High Performance Liquid Chromatography- Inorganic arsenic, Inductively Coupled Plasma-Mass Dimethylarsinic acid (DMA), EAM 4.10 Spectrometric Determination of Four Arsenic Monomethylarsonic acid (MMA), Species in Fruit Juice Arsenobetaine (AsB) KAN-LAB-MET.95 Determination of Iodine in Foods Iodine Methods for Radionuclides Method Method Description Analyte Determination of Strontium-90 in Foods by WEAC.RN.METHOD.2.0 Strontium-90 Internal Gas-Flow Proportional Counting Americium-241 Cesium-134 Cesium-137 Determination of Gamma-Ray Emitting Cobalt-60 WEAC.RN.METHOD.3.0 Radionuclides in Foods by High-Purity Potassium-40 Germanium Spectrometry Radium-226 Ruthenium-103 Ruthenium-106 Thorium-232 Methods for Pesticides/Industrial Chemicals Method Method Description Analyte Extraction Method: Analysis of Pesticides KAN-LAB-PES.53 and -

Genomic and Phenotypic Alterations of the Neuronal-Like Cells
Int. J. Mol. Sci. 2014, 15, 905-926; doi:10.3390/ijms15010905 OPEN ACCESS International Journal of Molecular Sciences ISSN 1422-0067 www.mdpi.com/journal/ijms Article Genomic and Phenotypic Alterations of the Neuronal-Like Cells Derived from Human Embryonal Carcinoma Stem Cells (NT2) Caused by Exposure to Organophosphorus Compounds Paraoxon and Mipafox David Pamies 1,2,3,*, Miguel A. Sogorb 1, Marco Fabbri 2,4, Laura Gribaldo 2, Angelo Collotta 2, Bibiana Scelfo 2, Eugenio Vilanova 1, Georgina Harris 2,3 and Anna Bal-Price 2 1 Bioengineering Institute, Miguel Hernández University, Elche 03202, Alicante, Spain; E-Mails: [email protected] (M.A.S.); [email protected] (E.V.) 2 Institute for Health and Consumer Protection, European Commission Joint Research Centre, Ispra, Varese 21027, Italy; E-Mails: [email protected] (M.F.); [email protected] (L.G.); [email protected] (A.C.); [email protected] (B.S.); [email protected] (G.H.); [email protected] (A.B.-P.) 3 Bloomberg School of Public Health, Johns Hopkins University, CAAT, Baltimore, MD 21205, USA 4 Department of Experimental and Clinical Medicine, University of Insubria, Varese 21100, Italy * Author to whom correspondence should be addressed; E-Mail: [email protected]; Tel.: +1-410-614-4990; Fax: +1-470-614-2871. Received: 14 October 2013; in revised form: 8 December 2013 / Accepted: 17 December 2013 / Published: 9 January 2014 Abstract: Historically, only few chemicals have been identified as neurodevelopmental toxicants, however, concern remains, and has recently increased, based upon the association between chemical exposures and increased developmental disorders.