A SAS-6-Like Protein Suggests That the Toxoplasma Conoid Complex Evolved from Flagellar Components Jessica Cruz De Leon University of California - Irvine
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Basal Body Structure and Composition in the Apicomplexans Toxoplasma and Plasmodium Maria E
Francia et al. Cilia (2016) 5:3 DOI 10.1186/s13630-016-0025-5 Cilia REVIEW Open Access Basal body structure and composition in the apicomplexans Toxoplasma and Plasmodium Maria E. Francia1* , Jean‑Francois Dubremetz2 and Naomi S. Morrissette3 Abstract The phylum Apicomplexa encompasses numerous important human and animal disease-causing parasites, includ‑ ing the Plasmodium species, and Toxoplasma gondii, causative agents of malaria and toxoplasmosis, respectively. Apicomplexans proliferate by asexual replication and can also undergo sexual recombination. Most life cycle stages of the parasite lack flagella; these structures only appear on male gametes. Although male gametes (microgametes) assemble a typical 9 2 axoneme, the structure of the templating basal body is poorly defined. Moreover, the rela‑ tionship between asexual+ stage centrioles and microgamete basal bodies remains unclear. While asexual stages of Plasmodium lack defined centriole structures, the asexual stages of Toxoplasma and closely related coccidian api‑ complexans contain centrioles that consist of nine singlet microtubules and a central tubule. There are relatively few ultra-structural images of Toxoplasma microgametes, which only develop in cat intestinal epithelium. Only a subset of these include sections through the basal body: to date, none have unambiguously captured organization of the basal body structure. Moreover, it is unclear whether this basal body is derived from pre-existing asexual stage centrioles or is synthesized de novo. Basal bodies in Plasmodium microgametes are thought to be synthesized de novo, and their assembly remains ill-defined. Apicomplexan genomes harbor genes encoding δ- and ε-tubulin homologs, potentially enabling these parasites to assemble a typical triplet basal body structure. -
Molecular Data and the Evolutionary History of Dinoflagellates by Juan Fernando Saldarriaga Echavarria Diplom, Ruprecht-Karls-Un
Molecular data and the evolutionary history of dinoflagellates by Juan Fernando Saldarriaga Echavarria Diplom, Ruprecht-Karls-Universitat Heidelberg, 1993 A THESIS SUBMITTED IN PARTIAL FULFILMENT OF THE REQUIREMENTS FOR THE DEGREE OF DOCTOR OF PHILOSOPHY in THE FACULTY OF GRADUATE STUDIES Department of Botany We accept this thesis as conforming to the required standard THE UNIVERSITY OF BRITISH COLUMBIA November 2003 © Juan Fernando Saldarriaga Echavarria, 2003 ABSTRACT New sequences of ribosomal and protein genes were combined with available morphological and paleontological data to produce a phylogenetic framework for dinoflagellates. The evolutionary history of some of the major morphological features of the group was then investigated in the light of that framework. Phylogenetic trees of dinoflagellates based on the small subunit ribosomal RNA gene (SSU) are generally poorly resolved but include many well- supported clades, and while combined analyses of SSU and LSU (large subunit ribosomal RNA) improve the support for several nodes, they are still generally unsatisfactory. Protein-gene based trees lack the degree of species representation necessary for meaningful in-group phylogenetic analyses, but do provide important insights to the phylogenetic position of dinoflagellates as a whole and on the identity of their close relatives. Molecular data agree with paleontology in suggesting an early evolutionary radiation of the group, but whereas paleontological data include only taxa with fossilizable cysts, the new data examined here establish that this radiation event included all dinokaryotic lineages, including athecate forms. Plastids were lost and replaced many times in dinoflagellates, a situation entirely unique for this group. Histones could well have been lost earlier in the lineage than previously assumed. -

University of Malaya Kuala Lumpur
GENETIC DIVERSITY STUDY, EXPRESSION AND IMMUNOCHARACTERIZATION OF PLASMODIUM KNOWLESI MEROZOITE SURFACE PROTEIN-3 (MSP-3) IN ESCHERICHIA COLI JEREMY RYAN DE SILVA THESIS SUBMITTED IN FULLFILMENT OF THE REQUIREMENTSMalaya FOR THE DEGREE OF DOCTOR OF PHILOSOPHY of FACULTY OF MEDICINE UNIVERSITY OF MALAYA KUALA LUMPUR University 2017 UNIVERSITI MALAYA ORIGINAL LITERARY WORK DECLARATION Name of Candidate : Jeremy Ryan De Silva Registration / Matric No : MHA120057 Name of Degree : Doctor Of Philosophy (Ph.D) Title of Project Paper / Research Report / Dissertation / Thesis (“this Work”): Genetic diversity study, expression and immunocharacterization of Plasmodium Knowlesi Merozoite Surface Protein-3 (MSP-3) in Escherichia Coli Field of Study : Medical Parasitology I do solemnly and sincerely declare that: [1] I am the sole author / writer of this Work; [2] This Work is original; [3] Any use of any work in which copyright exists was done by way of fair dealing and for permitted purposes and any excerpt or extract from, or reference to or reproduction of any copyright work has been disclosed expressly and sufficiently and the title ofMalaya the Work and its authorship have been acknowledged in this Work; [4] I do not have any actual knowledge nor do I ought reasonably to know that the making of this work constitutes an infringement of any copyright work; [5] I hereby assign all and every rights in the copyrightof to this Work to the University of Malaya (“UM”), who henceforth shall be owner of the copyright in this Work and that any reproduction or use in any form or by any means whatsoever is prohibited without the written consent of UM having been first had and obtained; [6] I am fully aware that if in the course of making this Work I have infringed any copyright whether intentionally or otherwise, I may be subject to legal action or any other action as may be determined by UM. -

Coccidiosis in Large and Small Ruminants
Coccidiosis in Large and Small Ruminants a, b Sarah Tammy Nicole Keeton, PhD, MS *, Christine B. Navarre, DVM, MS KEYWORDS Coccidia Coccidiosis Diarrhea Ruminants Cattle Sheep Goats Ionophores KEY POINTS Coccidiosis is an important parasitic disease of ruminant livestock caused by the proto- zoan parasite of the genus Eimeria. Calves between 6 and 12 months of age and lambs and kids between 1 and 6 months of age are most susceptible. Subclinical disease is characterized by poor growth. Clinical disease is most commonly characterized by diarrhea. Control of coccidiosis is based on sound management, the use of preventive medications, and treatment of clinical cases as necessary. INTRODUCTION: NATURE OF THE PROBLEM Coccidiosis is a parasitic disease of vertebrate animals, including domestic ruminants.1 It is economically significant, with losses from both clinical and subclinical disease. Coccidiosis is caused by the protozoan parasite of the genus Eimeria. Eimeria are host specific, meaning that an Eimeria species that infect goats does not infect sheep or cattle and vice versa. Certain species of Eimeria are nonpathogenic and do not cause disease. The pathogenic species and sites of infection are listed in Table 1. Mixed infections with multiple pathogenic and nonpathogenic species is common. LIFE CYCLE Proper treatment and control of coccidiosis requires an understanding of the complex life cycle and transmission of Eimeria spp (Fig. 1). The life cycle can be divided into Disclosure: The authors have nothing to disclose. a Department of Veterinary Clinical Sciences, School of Veterinary Medicine, Louisiana State University, Skip Bertman Drive, Baton Rouge, LA 70803, USA; b LSU AgCenter, School of Animal Sciences, Louisiana State University, 111 Dalrymple Bldg, 110 LSU Union Square, Baton Rouge, LA 70803-0106, USA * Corresponding author. -

Protocols for Monitoring Harmful Algal Blooms for Sustainable Aquaculture and Coastal Fisheries in Chile (Supplement Data)
Protocols for monitoring Harmful Algal Blooms for sustainable aquaculture and coastal fisheries in Chile (Supplement data) Provided by Kyoko Yarimizu, et al. Table S1. Phytoplankton Naming Dictionary: This dictionary was constructed from the species observed in Chilean coast water in the past combined with the IOC list. Each name was verified with the list provided by IFOP and online dictionaries, AlgaeBase (https://www.algaebase.org/) and WoRMS (http://www.marinespecies.org/). The list is subjected to be updated. Phylum Class Order Family Genus Species Ochrophyta Bacillariophyceae Achnanthales Achnanthaceae Achnanthes Achnanthes longipes Bacillariophyta Coscinodiscophyceae Coscinodiscales Heliopeltaceae Actinoptychus Actinoptychus spp. Dinoflagellata Dinophyceae Gymnodiniales Gymnodiniaceae Akashiwo Akashiwo sanguinea Dinoflagellata Dinophyceae Gymnodiniales Gymnodiniaceae Amphidinium Amphidinium spp. Ochrophyta Bacillariophyceae Naviculales Amphipleuraceae Amphiprora Amphiprora spp. Bacillariophyta Bacillariophyceae Thalassiophysales Catenulaceae Amphora Amphora spp. Cyanobacteria Cyanophyceae Nostocales Aphanizomenonaceae Anabaenopsis Anabaenopsis milleri Cyanobacteria Cyanophyceae Oscillatoriales Coleofasciculaceae Anagnostidinema Anagnostidinema amphibium Anagnostidinema Cyanobacteria Cyanophyceae Oscillatoriales Coleofasciculaceae Anagnostidinema lemmermannii Cyanobacteria Cyanophyceae Oscillatoriales Microcoleaceae Annamia Annamia toxica Cyanobacteria Cyanophyceae Nostocales Aphanizomenonaceae Aphanizomenon Aphanizomenon flos-aquae -

Texto Completo
Ministério da Saúde Fundação Oswaldo Cruz Centro de Pesquisas René Rachou Programa de Pós-Graduação em Ciências da Saúde Estudo Molecular dos Parasitos Causadores da Malária Simiana no Centro de Primatologia do Rio de Janeiro, Brasil por Denise Anete Madureira de Alvarenga Belo Horizonte Dezembro/2014 DISSERTAÇÃO MBCM-CPqRR D.A.M. ALVARENGA 2014 Alvarenga, DAM Ministério da Saúde Fundação Oswaldo Cruz Centro de Pesquisas René Rachou Programa de Pós-Graduação em Ciências da Saúde Estudo Molecular dos Parasitos Causadores da Malária Simiana no Centro de Primatologia do Rio de Janeiro, Brasil por Denise Anete Madureira de Alvarenga Dissertação apresentada com vistas à obtenção do Título de Mestre em Ciências na área de concentração Biologia Celular e Molecular. Orientação: Dra. Cristiana Ferreira Alves de Brito Co-orientação: Dra. Taís Nóbrega de Sousa Belo Horizonte Dezembro/2014 Alvarenga, DAM II Catalogação-na-fonte Rede de Bibliotecas da FIOCRUZ Biblioteca do CPqRR Segemar Oliveira Magalhães CRB/6 1975 A473e 2014 Alvarenga, Denise Anete Madureira. Estudo Molecular dos Parasitos Causadores da Malária Simiana no Centro de Primatologia do Rio de Janeiro, Brasil / Denise Anete Madureira de Alvarenga. – Belo Horizonte, 2014. XXI, 58 f.: il.; 210 x 297mm Bibliografia: f. 70 - 77 Dissertação (mestrado) – Dissertação para obtenção do título de Mestre em Ciências pelo Programa de Pós- Graduação em Ciências da Saúde do Centro de Pesquisas René Rachou. Área de concentração: Biologia Celular e Molecular. 1. Malária Vivax/genética 2. Plasmodium vivax /imunologia 3. Reservatórios de Doenças/classificação I. Título. II. Brito, Cristiana Ferreira Alves (Orientação). III. Souza, Taís Nóbrega (Co-orientação) CDD – 22. -

Ana Júlia Dutra Nunes Prevalência De Infecção
ANA JÚLIA DUTRA NUNES PREVALÊNCIA DE INFECÇÃO POR Plasmodium spp. E SUA ASSOCIAÇÃO COM OS PARÂMETROS BIOQUÍMICOS E HEMATOLÓGICOS DE Alouatta guariba clamitans (CABRERA, 1940) (PRIMATES: ATELIDAE) DE VIDA LIVRE JOINVILLE, 2019 ANA JÚLIA DUTRA NUNES PREVALÊNCIA DE INFECÇÃO POR Plasmodium spp. E SUA ASSOCIAÇÃO COM OS PARÂMETROS BIOQUÍMICOS E HEMATOLÓGICOS DE Alouatta guariba clamitans (CABRERA, 1940) (PRIMATES: ATELIDAE) DE VIDA LIVRE. Dissertação de mestrado apresentada como requisito parcial para obtenção do título de Mestre em Saúde e Meio Ambiente, na Universidade da Região de Joinville. Orientadora: Dra. Marta Jussara Cremer. Coorientadora: Dra. Cristiana Ferreira Alves de Brito. JOINVILLE, 2019 Catalogação na publicação pela Biblioteca Universitária da Univille Nunes, Ana Júlia Dutra N972p Prevalência de infecção por Plasmodium spp. e sua associação com os parâmetros bioquímicos e hematológicos de Alouatta guariba clamitans (Cabrera, 1940) (Primates: Atelidae) de vida livre. / Ana Júlia Dutra Nunes; orientadora Dra. Marta Jussara Cremer, coorientadora Dra. Cristiana Ferreira Alves de Brito. – Joinville: UNIVILLE, 2019. 65 p.: il. ; 30 cm Dissertação (Mestrado em Saúde e Meio Ambiente – Universidade da Região de Joinville) 1. Alouatta guariba clamitans Cabrera. 2. Malária. 3. Conservação de espécies. I. Cremer, Marta Jussara (orient.). II. Brito, Cristiana Ferreira Alves de (coord.). III. Título. CDD 636.200896951 Elaborada por Christiane de Viveiros Cardozo – CRB-14/778 Termo de Aprovação "Prevalência da Infecção por Plasmodium spp e sua Associação com os Parâmetros Bioquímicos e Hematológicos de Alouatta guariba clamitans (Cabrera, 1940) (Primates: Atelidae) de Vida Livre" por Ana Júlia Dutra Nunes Dissertação julgada para a obtenção do título de Mestra em Saúde e Meio Ambiente, área de concentração Saúde e Meio Ambiente e aprovada em sua forma final pelo Programa de Pós- Graduação em Saúde e Meio Ambiente. -
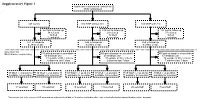
Supplementary Figure 1 Multicenter Randomised Control Trial 2746 Randomised
Supplementary Figure 1 Multicenter randomised control trial 2746 randomised 947 control 910 MNP without zinc 889 MNP with zinc 223 lost to follow up 219 lost to follow up 183 lost to follow up 34 refused 29 refused 37 refused 16 died 12 died 9 died 3 excluded 4 excluded 1 excluded 671 in follow-up 646 in follow-up 659 in follow-up at 24mo of age at 24mo of age at 24mo of age Selection for Microbiome sequencing 516 paired samples unavailable 469 paired samples unavailable 497 paired samples unavailable 69 antibiotic use 63 antibiotic use 67 antibiotic use 31 outside of WLZ criteria 37 outside of WLZ criteria 34 outside of WLZ criteria 6 diarrhea last 7 days 2 diarrhea last 7 days 7 diarrhea last 7 days 39 WLZ > -1 at 24 mo 10 WLZ < -2 at 24mo 58 WLZ > -1 at 24 mo 17 WLZ < -2 at 24mo 48 WLZ > -1 at 24 mo 8 WLZ < -2 at 24mo available for selection available for selection available for selection available for selection available for selection1 available for selection1 14 selected 10 selected 15 selected 14 selected 20 selected1 7 selected1 1 Two subjects (one in the reference WLZ group and one undernourished) had, at 12 months, no diarrhea within 1 day of stool collection but reported diarrhea within 7 days prior. Length, cm kg Weight, Supplementary Figure 2. Length (left) and weight (right) z-scores of children recruited into clinical trial NCT00705445 during the first 24 months of life. Median and quantile values are shown, with medians for participants profiled in current study indicated by red (undernourished) and black (reference WLZ) lines. -

Ellobiopsids of the Genus Thalassomyces Are Alveolates
J. Eukaryot. Microbiol., 51(2), 2004 pp. 246±252 q 2004 by the Society of Protozoologists Ellobiopsids of the Genus Thalassomyces are Alveolates JEFFREY D. SILBERMAN,a,b1 ALLEN G. COLLINS,c,2 LISA-ANN GERSHWIN,d,3 PATRICIA J. JOHNSONa and ANDREW J. ROGERe aDepartment of Microbiology, Immunology, and Molecular Genetics, University of California at Los Angeles, California, USA, and bInstitute of Geophysics and Planetary Physics, University of California at Los Angeles, California, USA, and cEcology, Behavior and Evolution Section, Division of Biology, University of California, La Jolla, California, USA, and dDepartment of Integrative Biology and Museum of Paleontology, University of California, Berkeley, California, USA, and eCanadian Institute for Advanced Research, Program in Evolutionary Biology, Genome Atlantic, Department of Biochemistry and Molecular Biology, Dalhousie University, Halifax, Nova Scotia, Canada ABSTRACT. Ellobiopsids are multinucleate protist parasites of aquatic crustaceans that possess a nutrient absorbing `root' inside the host and reproductive structures that protrude through the carapace. Ellobiopsids have variously been af®liated with fungi, `colorless algae', and dino¯agellates, although no morphological character has been identi®ed that de®nitively allies them with any particular eukaryotic lineage. The arrangement of the trailing and circumferential ¯agella of the rarely observed bi-¯agellated `zoospore' is reminiscent of dino¯agellate ¯agellation, but a well-organized `dinokaryotic nucleus' has never been observed. Using small subunit ribosomal RNA gene sequences from two species of Thalassomyces, phylogenetic analyses robustly place these ellobiopsid species among the alveolates (ciliates, apicomplexans, dino¯agellates and relatives) though without a clear af®liation to any established alveolate lineage. Our trees demonstrate that Thalassomyces fall within a dino¯agellate 1 apicomplexa 1 Perkinsidae 1 ``marine alveolate group 1'' clade, clustering most closely with dino¯agellates. -

Diversity of Extracellular Proteins During the Transition from the 'Proto
1 Diversity of extracellular proteins during the transition from the ‘proto-apicomplexan’ alveolates to the apicomplexan obligate parasites THOMAS J. TEMPLETON1,2* and ARNAB PAIN3,4 1 Department of Protozoology, Institute of Tropical Medicine (NEKKEN), Nagasaki University, 1-12-4 Sakamoto, Nagasaki 852-8523, Japan 2 Department of Microbiology and Immunology, Weill Cornell Medical College, New York 10021, USA 3 Pathogen Genomics Laboratory, Biological and Environmental Sciences and Engineering (BESE) Division, King Abdullah University of Science and Technology (KAUST), Thuwal, Jeddah 23955-6900, Kingdom of Saudi Arabia 4 Global Station for Zoonosis Control, Global Institution for Collaborative Research and Education (GI-CoRE), Hokkaido University, N20 W10 Kita-ku, Sapporo 001-0020, Japan (Received 22 May 2015; revised 12 August 2015; accepted 14 August 2015) SUMMARY The recent completion of high-coverage draft genome sequences for several alveolate protozoans – namely, the chromer- ids, Chromera velia and Vitrella brassicaformis; the perkinsid Perkinsus marinus; the apicomplexan, Gregarina niphandrodes, as well as high coverage transcriptome sequence information for several colpodellids, allows for new genome-scale com- parisons across a rich landscape of apicomplexans and other alveolates. Genome annotations can now be used to help in- terpret fine ultrastructure and cell biology, and guide new studies to describe a variety of alveolate life strategies, such as symbiosis or free living, predation, and obligate intracellular parasitism, as well to provide foundations to dissect the evo- lutionary transitions between these niches. This review focuses on the attempt to identify extracellular proteins which might mediate the physical interface of cell–cell interactions within the above life strategies, aided by annotation of the repertoires of predicted surface and secreted proteins encoded within alveolate genomes. -

Molecular Phylogeny and Surface Morphology of Colpodella Edax (Alveolata): Insights Into the Phagotrophic Ancestry of Apicomplexans
J. Eukaryot. Microbiol., 50(5), 2003 pp. 334±340 q 2003 by the Society of Protozoologists Molecular Phylogeny and Surface Morphology of Colpodella edax (Alveolata): Insights into the Phagotrophic Ancestry of Apicomplexans BRIAN S. LEANDER,a OLGA N. KUVARDINA,b VLADIMIR V. ALESHIN,b ALEXANDER P. MYLNIKOVc and PATRICK J. KEELINGa aCanadian Institute for Advanced Research, Program in Evolutionary Biology, Department of Botany, University of British Columbia, Vancouver, BC, V6T 1Z4, Canada, and bDepartments of Evolutionary Biochemistry and Invertebrate Zoology, Belozersky Institute of Physico-Chemical Biology, Moscow State University, Moscow, 119 992, Russian Federation, and cInstitute for the Biology of Inland Waters, Russian Academy of Sciences, Borok, Yaroslavskaya oblast, 152742, Russian Federation ABSTRACT. The molecular phylogeny of colpodellids provides a framework for inferences about the earliest stages in apicomplexan evolution and the characteristics of the last common ancestor of apicomplexans and dino¯agellates. We extended this research by presenting phylogenetic analyses of small subunit rRNA gene sequences from Colpodella edax and three unidenti®ed eukaryotes published from molecular phylogenetic surveys of anoxic environments. Phylogenetic analyses consistently showed C. edax and the environmental sequences nested within a colpodellid clade, which formed the sister group to (eu)apicomplexans. We also presented surface details of C. edax using scanning electron microscopy in order to supplement previous ultrastructural investigations of this species using transmission electron microscopy and to provide morphological context for interpreting environmental sequences. The microscopical data con®rmed a sparse distribution of micropores, an amphiesma consisting of small polygonal alveoli, ¯agellar hairs on the anterior ¯agellum, and a rostrum molded by the underlying (open-sided) conoid. -

Genetic Diversity and Habitats of Two Enigmatic Marine Alveolate Lineages
AQUATIC MICROBIAL ECOLOGY Vol. 42: 277–291, 2006 Published March 29 Aquat Microb Ecol Genetic diversity and habitats of two enigmatic marine alveolate lineages Agnès Groisillier1, Ramon Massana2, Klaus Valentin3, Daniel Vaulot1, Laure Guillou1,* 1Station Biologique, UMR 7144, CNRS, and Université Pierre & Marie Curie, BP74, 29682 Roscoff Cedex, France 2Department de Biologia Marina i Oceanografia, Institut de Ciències del Mar, CMIMA, CSIC. Passeig Marítim de la Barceloneta 37-49, 08003 Barcelona, Spain 3Alfred Wegener Institute for Polar Research, Am Handelshafen 12, 27570 Bremerhaven, Germany ABSTRACT: Systematic sequencing of environmental SSU rDNA genes amplified from different marine ecosystems has uncovered novel eukaryotic lineages, in particular within the alveolate and stramenopile radiations. The ecological and geographic distribution of 2 novel alveolate lineages (called Group I and II in previous papers) is inferred from the analysis of 62 different environmental clone libraries from freshwater and marine habitats. These 2 lineages have been, up to now, retrieved exclusively from marine ecosystems, including oceanic and coastal waters, sediments, hydrothermal vents, and perma- nent anoxic deep waters and usually represent the most abundant eukaryotic lineages in environmen- tal genetic libraries. While Group I is only composed of environmental sequences (118 clones), Group II contains, besides environmental sequences (158 clones), sequences from described genera (8) (Hema- todinium and Amoebophrya) that belong to the Syndiniales, an atypical order of dinoflagellates exclu- sively composed of marine parasites. This suggests that Group II could correspond to Syndiniales, al- though this should be confirmed in the future by examining the morphology of cells from Group II. Group II appears to be abundant in coastal and oceanic ecosystems, whereas permanent anoxic waters and hy- drothermal ecosystems are usually dominated by Group I.