Basic Physics of X-Ray Imaging
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Lecture 6: Spectroscopy and Photochemistry II
Lecture 6: Spectroscopy and Photochemistry II Required Reading: FP Chapter 3 Suggested Reading: SP Chapter 3 Atmospheric Chemistry CHEM-5151 / ATOC-5151 Spring 2005 Prof. Jose-Luis Jimenez Outline of Lecture • The Sun as a radiation source • Attenuation from the atmosphere – Scattering by gases & aerosols – Absorption by gases • Beer-Lamber law • Atmospheric photochemistry – Calculation of photolysis rates – Radiation fluxes – Radiation models 1 Reminder of EM Spectrum Blackbody Radiation Linear Scale Log Scale From R.P. Turco, Earth Under Siege: From Air Pollution to Global Change, Oxford UP, 2002. 2 Solar & Earth Radiation Spectra • Sun is a radiation source with an effective blackbody temperature of about 5800 K • Earth receives circa 1368 W/m2 of energy from solar radiation From Turco From S. Nidkorodov • Question: are relative vertical scales ok in right plot? Solar Radiation Spectrum II From F-P&P •Solar spectrum is strongly modulated by atmospheric scattering and absorption From Turco 3 Solar Radiation Spectrum III UV Photon Energy ↑ C B A From Turco Solar Radiation Spectrum IV • Solar spectrum is strongly O3 modulated by atmospheric absorptions O 2 • Remember that UV photons have most energy –O2 absorbs extreme UV in mesosphere; O3 absorbs most UV in stratosphere – Chemistry of those regions partially driven by those absorptions – Only light with λ>290 nm penetrates into the lower troposphere – Biomolecules have same bonds (e.g. C-H), bonds can break with UV absorption => damage to life • Importance of protection From F-P&P provided by O3 layer 4 Solar Radiation Spectrum vs. altitude From F-P&P • Very high energy photons are depleted high up in the atmosphere • Some photochemistry is possible in stratosphere but not in troposphere • Only λ > 290 nm in trop. -

Section 1: Introduction (PDF)
SECTION 1: Introduction SECTION 1: INTRODUCTION Section 1 Contents The Purpose and Scope of This Guidance ....................................................................1-1 Relationship to CZARA Guidance ....................................................................................1-2 National Water Quality Inventory .....................................................................................1-3 What is Nonpoint Source Pollution? ...............................................................................1-4 Watershed Approach to Nonpoint Source Pollution Control .......................................1-5 Programs to Control Nonpoint Source Pollution...........................................................1-7 National Nonpoint Source Pollution Control Program .............................................1-7 Storm Water Permit Program .......................................................................................1-8 Coastal Nonpoint Pollution Control Program ............................................................1-8 Clean Vessel Act Pumpout Grant Program ................................................................1-9 International Convention for the Prevention of Pollution from Ships (MARPOL)...................................................................................................1-9 Oil Pollution Act (OPA) and Regulation ....................................................................1-10 Sources of Further Information .....................................................................................1-10 -

Sources and Emissions of Air Pollutants
© Jones & Bartlett Learning, LLC. NOT FOR SALE OR DISTRIBUTION Chapter 2 Sources and Emissions of Air Pollutants LEArning ObjECtivES By the end of this chapter the reader will be able to: • distinguish the “troposphere” from the “stratosphere” • define “polluted air” in relation to various scientific disciplines • describe “anthropogenic” sources of air pollutants and distinguish them from “natural” sources • list 10 sources of indoor air contaminants • identify three meteorological factors that affect the dispersal of air pollutants ChAPtEr OutLinE I. Introduction II. Measurement Basics III. Unpolluted vs. Polluted Air IV. Air Pollutant Sources and Their Emissions V. Pollutant Transport VI. Summary of Major Points VII. Quiz and Problems VIII. Discussion Topics References and Recommended Reading 21 2 N D R E V I S E 9955 © Jones & Bartlett Learning, LLC. NOT FOR SALE OR DISTRIBUTION 22 Chapter 2 SourCeS and EmiSSionS of Air PollutantS i. IntrOduCtiOn level. Mt. Everest is thus a minute bump on the globe that adds only 0.06 percent to the Earth’s diameter. Structure of the Earth’s Atmosphere The Earth’s atmosphere consists of several defined layers (Figure 2–1). The troposphere, in which all life The Earth, along with Mercury, Venus, and Mars, is exists, and from which we breathe, reaches an altitude of a terrestrial (as opposed to gaseous) planet with a per- about 7–8 km at the poles to just over 13 km at the equa- manent atmosphere. The Earth is an oblate (slightly tor: the mean thickness being 9.1 km (5.7 miles). Thus, flattened) sphere with a mean diameter of 12,700 km the troposphere represents a very thin cover over the (about 8,000 statute miles). -

7. Gamma and X-Ray Interactions in Matter
Photon interactions in matter Gamma- and X-Ray • Compton effect • Photoelectric effect Interactions in Matter • Pair production • Rayleigh (coherent) scattering Chapter 7 • Photonuclear interactions F.A. Attix, Introduction to Radiological Kinematics Physics and Radiation Dosimetry Interaction cross sections Energy-transfer cross sections Mass attenuation coefficients 1 2 Compton interaction A.H. Compton • Inelastic photon scattering by an electron • Arthur Holly Compton (September 10, 1892 – March 15, 1962) • Main assumption: the electron struck by the • Received Nobel prize in physics 1927 for incoming photon is unbound and stationary his discovery of the Compton effect – The largest contribution from binding is under • Was a key figure in the Manhattan Project, condition of high Z, low energy and creation of first nuclear reactor, which went critical in December 1942 – Under these conditions photoelectric effect is dominant Born and buried in • Consider two aspects: kinematics and cross Wooster, OH http://en.wikipedia.org/wiki/Arthur_Compton sections http://www.findagrave.com/cgi-bin/fg.cgi?page=gr&GRid=22551 3 4 Compton interaction: Kinematics Compton interaction: Kinematics • An earlier theory of -ray scattering by Thomson, based on observations only at low energies, predicted that the scattered photon should always have the same energy as the incident one, regardless of h or • The failure of the Thomson theory to describe high-energy photon scattering necessitated the • Inelastic collision • After the collision the electron departs -

Photon Cross Sections, Attenuation Coefficients, and Energy Absorption Coefficients from 10 Kev to 100 Gev*
1 of Stanaaros National Bureau Mmin. Bids- r'' Library. Ml gEP 2 5 1969 NSRDS-NBS 29 . A111D1 ^67174 tioton Cross Sections, i NBS Attenuation Coefficients, and & TECH RTC. 1 NATL INST OF STANDARDS _nergy Absorption Coefficients From 10 keV to 100 GeV U.S. DEPARTMENT OF COMMERCE NATIONAL BUREAU OF STANDARDS T X J ". j NATIONAL BUREAU OF STANDARDS 1 The National Bureau of Standards was established by an act of Congress March 3, 1901. Today, in addition to serving as the Nation’s central measurement laboratory, the Bureau is a principal focal point in the Federal Government for assuring maximum application of the physical and engineering sciences to the advancement of technology in industry and commerce. To this end the Bureau conducts research and provides central national services in four broad program areas. These are: (1) basic measurements and standards, (2) materials measurements and standards, (3) technological measurements and standards, and (4) transfer of technology. The Bureau comprises the Institute for Basic Standards, the Institute for Materials Research, the Institute for Applied Technology, the Center for Radiation Research, the Center for Computer Sciences and Technology, and the Office for Information Programs. THE INSTITUTE FOR BASIC STANDARDS provides the central basis within the United States of a complete and consistent system of physical measurement; coordinates that system with measurement systems of other nations; and furnishes essential services leading to accurate and uniform physical measurements throughout the Nation’s scientific community, industry, and com- merce. The Institute consists of an Office of Measurement Services and the following technical divisions: Applied Mathematics—Electricity—Metrology—Mechanics—Heat—Atomic and Molec- ular Physics—Radio Physics -—Radio Engineering -—Time and Frequency -—Astro- physics -—Cryogenics. -

Chapter 30: Quantum Physics ( ) ( )( ( )( ) ( )( ( )( )
Chapter 30: Quantum Physics 9. The tungsten filament in a standard light bulb can be considered a blackbody radiator. Use Wien’s Displacement Law (equation 30-1) to find the peak frequency of the radiation from the tungsten filament. −− 10 1 1 1. (a) Solve Eq. 30-1 fTpeak =×⋅(5.88 10 s K ) for the peak frequency: =×⋅(5.88 1010 s−− 1 K 1 )( 2850 K) =× 1.68 1014 Hz 2. (b) Because the peak frequency is that of infrared electromagnetic radiation, the light bulb radiates more energy in the infrared than the visible part of the spectrum. 10. The image shows two oxygen atoms oscillating back and forth, similar to a mass on a spring. The image also shows the evenly spaced energy levels of the oscillation. Use equation 13-11 to calculate the period of oscillation for the two atoms. Calculate the frequency, using equation 13-1, from the inverse of the period. Multiply the period by Planck’s constant to calculate the spacing of the energy levels. m 1. (a) Set the frequency T = 2π k equal to the inverse of the period: 1 1k 1 1215 N/m f = = = =4.792 × 1013 Hz Tm22ππ1.340× 10−26 kg 2. (b) Multiply the frequency by Planck’s constant: E==×⋅ hf (6.63 10−−34 J s)(4.792 × 1013 Hz) =× 3.18 1020 J 19. The light from a flashlight can be considered as the emission of many photons of the same frequency. The power output is equal to the number of photons emitted per second multiplied by the energy of each photon. -

The Basic Interactions Between Photons and Charged Particles With
Outline Chapter 6 The Basic Interactions between • Photon interactions Photons and Charged Particles – Photoelectric effect – Compton scattering with Matter – Pair productions Radiation Dosimetry I – Coherent scattering • Charged particle interactions – Stopping power and range Text: H.E Johns and J.R. Cunningham, The – Bremsstrahlung interaction th physics of radiology, 4 ed. – Bragg peak http://www.utoledo.edu/med/depts/radther Photon interactions Photoelectric effect • Collision between a photon and an • With energy deposition atom results in ejection of a bound – Photoelectric effect electron – Compton scattering • The photon disappears and is replaced by an electron ejected from the atom • No energy deposition in classical Thomson treatment with kinetic energy KE = hν − Eb – Pair production (above the threshold of 1.02 MeV) • Highest probability if the photon – Photo-nuclear interactions for higher energies energy is just above the binding energy (above 10 MeV) of the electron (absorption edge) • Additional energy may be deposited • Without energy deposition locally by Auger electrons and/or – Coherent scattering Photoelectric mass attenuation coefficients fluorescence photons of lead and soft tissue as a function of photon energy. K and L-absorption edges are shown for lead Thomson scattering Photoelectric effect (classical treatment) • Electron tends to be ejected • Elastic scattering of photon (EM wave) on free electron o at 90 for low energy • Electron is accelerated by EM wave and radiates a wave photons, and approaching • No -

Photons Outline
1 Photons Observational Astronomy 2019 Part 1 Prof. S.C. Trager 2 Outline Wavelengths, frequencies, and energies of photons The EM spectrum Fluxes, filters, magnitudes & colors 3 Wavelengths, frequencies, and energies of photons Recall that λν=c, where λ is the wavelength of a photon, ν is its frequency, and c is the speed of light in a vacuum, c=2.997925×1010 cm s–1 The human eye is sensitive to wavelengths from ~3900 Å (1 Å=0.1 nm=10–8 cm=10–10 m) – blue light – to ~7200 Å – red light 4 “Optical” astronomy runs from ~3100 Å (the atmospheric cutoff) to ~1 µm (=1000 nm=10000 Å) Optical astronomers often refer to λ>8000 Å as “near- infrared” (NIR) – because it’s beyond the wavelength sensitivity of most people’s eyes – although NIR typically refers to the wavelength range ~1 µm to ~2.5 µm We’ll come back to this in a minute! 5 The energy of a photon is E=hν, where h=6.626×10–27 erg s is Planck’s constant High-energy (extreme UV, X-ray, γ-ray) astronomers often use eV (electron volt) as an energy unit, where 1 eV=1.602176×10–12 erg 6 Some useful relations: E (erg) 14 ⌫ (Hz) = 1 =2.418 10 E (eV) h (erg s− ) ⇥ ⇥ c hc 1 1 1 λ (A)˚ = = = 12398.4 E− (eV− ) ⌫ ⌫ E (eV) ⇥ Therefore a photon with a wavelength of 10 Å has an energy of ≈1.24 keV 7 If a photon was emitted from a blackbody of temperature T, then the average photon energy is Eav~kT, where k = 1.381×10–16 erg K–1 = 8.617×10–5 eV K–1 is Boltzmann’s constant. -
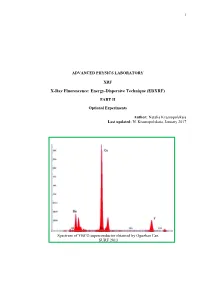
Advanced Physics Laboratory
1 ADVANCED PHYSICS LABORATORY XRF X-Ray Fluorescence: Energy-Dispersive Technique (EDXRF) PART II Optional Experiments Author: Natalia Krasnopolskaia Last updated: N. Krasnopolskaia, January 2017 Spectrum of YBCO superconductor obtained by Oguzhan Can. SURF 2013 2 Contents Introduction ………………………………………………………………………………………3 Experiment 1. Quantitative elemental analysis of coins ……………..………………………….12 Experiment 2. Study of Si PIN detector in the x-ray fluorescence spectrometer ……………….16 Experiment 3. Secondary enhancement of x-ray fluorescence and matrix effects in alloys.……24 Experiment 4. Verification of stoichiometry formula for superconducting materials..………….29 3 INTRODUCTION In Part I of the experiment you got familiar with fundamental ideas about the origin of x-ray photons as a result of decelerating of electrons in an x-ray tube and photoelectric effect in atoms of the x-ray tube and a target. For practical purposes, the processes in the x-ray tube and in the sample of interest must be explained in detail. An X-ray photon from the x-ray tube incident on a target/sample can be either absorbed by the atoms of the target or scattered. The corresponding processes are: (a) photoelectric effect (absorption) that gives rise to characteristic spectrum emitted isotropically; (b) Compton effect (inelastic scattering; energy shifts toward the lower value); and (c) Rayleigh scattering (elastic scattering; energy of the photon stays unchanged). Each event of interaction between the incident particle and an atom or the other particle is characterized by its probability, which in spectroscopy is expressed through the cross section σ of the process. Actually, σ has a dimension of [L2] and in atomic and nuclear physics has a unit of barn: 1 barn = 10-24cm2. -

Remote Sensing
remote sensing Article A Refined Four-Stream Radiative Transfer Model for Row-Planted Crops Xu Ma 1, Tiejun Wang 2 and Lei Lu 1,3,* 1 College of Earth and Environmental Sciences, Lanzhou University, Lanzhou 730000, China; [email protected] 2 Faculty of Geo-Information Science and Earth Observation (ITC), University of Twente, P.O. Box 217, 7500 AE Enschede, The Netherlands; [email protected] 3 Key Laboratory of Western China’s Environmental Systems (Ministry of Education), Lanzhou University, Lanzhou 730000, China * Correspondence: [email protected]; Tel.: +86-151-1721-8663 Received: 17 March 2020; Accepted: 14 April 2020; Published: 18 April 2020 Abstract: In modeling the canopy reflectance of row-planted crops, neglecting horizontal radiative transfer may lead to an inaccurate representation of vegetation energy balance and further cause uncertainty in the simulation of canopy reflectance at larger viewing zenith angles. To reduce this systematic deviation, here we refined the four-stream radiative transfer equations by considering horizontal radiation through the lateral “walls”, considered the radiative transfer between rows, then proposed a modified four-stream (MFS) radiative transfer model using single and multiple scattering. We validated the MFS model using both computer simulations and in situ measurements, and found that the MFS model can be used to simulate crop canopy reflectance at different growth stages with an accuracy comparable to the computer simulations (RMSE < 0.002 in the red band, RMSE < 0.019 in NIR band). Moreover, the MFS model can be successfully used to simulate the reflectance of continuous (RMSE = 0.012) and row crop canopies (RMSE < 0.023), and therefore addressed the large viewing zenith angle problems in the previous row model based on four-stream radiative transfer equations. -

DOE-HDBK-1122-99; Radiological Control Technician Training
DOE-HDBK-1122-99 Module 1.11 External Exposure Control Study Guide Course Title: Radiological Control Technician Module Title: External Exposure Control Module Number: 1.11 Objectives: 1.11.01 Identify the four basic methods for minimizing personnel external exposure. 1.11.02 Using the Exposure Rate = 6CEN equation, calculate the gamma exposure rate for specific radionuclides. 1.11.03 Identify "source reduction" techniques for minimizing personnel external exposures. 1.11.04 Identify "time-saving" techniques for minimizing personnel external exposures. 1.11.05 Using the stay time equation, calculate an individual's remaining allowable dose equivalent or stay time. 1.11.06 Identify "distance to radiation sources" techniques for minimizing personnel external exposures. 1.11.07 Using the point source equation (inverse square law), calculate the exposure rate or distance for a point source of radiation. 1.11.08 Using the line source equation, calculate the exposure rate or distance for a line source of radiation. 1.11.09 Identify how exposure rate varies depending on the distance from a surface (plane) source of radiation, and identify examples of plane sources. 1.11.10 Identify the definition and units of "mass attenuation coefficient" and "linear attenuation coefficient". 1.11.11 Identify the definition and units of "density thickness." 1.11.12 Identify the density-thickness values, in mg/cm2, for the skin, the lens of the eye and the whole body. 1.11.13 Calculate shielding thickness or exposure rates for gamma/x-ray radiation using the equations. 1.11-1 DOE-HDBK-1122-99 Module 1.11 External Exposure Control Study Guide INTRODUCTION The external exposure reduction and control measures available are of primary importance to the everyday tasks performed by the RCT. -

The Optical Effective Attenuation Coefficient As an Informative
hv photonics Article The Optical Effective Attenuation Coefficient as an Informative Measure of Brain Health in Aging Antonio M. Chiarelli 1,2,* , Kathy A. Low 1, Edward L. Maclin 1, Mark A. Fletcher 1, Tania S. Kong 1,3, Benjamin Zimmerman 1, Chin Hong Tan 1,4,5 , Bradley P. Sutton 1,6, Monica Fabiani 1,3,* and Gabriele Gratton 1,3,* 1 Beckman Institute for Advanced Science and Technology, University of Illinois at Urbana-Champaign, Urbana, IL 61801, USA 2 Department of Neuroscience, Imaging and Clinical Sciences, University G. D’Annunzio of Chieti-Pescara, 66100 Chieti, Italy 3 Psychology Department, University of Illinois at Urbana-Champaign, Champaign, IL 61820, USA 4 Division of Psychology, Nanyang Technological University, Singapore 639818, Singapore 5 Department of Pharmacology, National University of Singapore, Singapore 117600, Singapore 6 Department of Bioengineering, University of Illinois at Urbana-Champaign, Urbana, IL 61801, USA * Correspondence: [email protected] (A.M.C.); [email protected] (M.F.); [email protected] (G.G.) Received: 24 May 2019; Accepted: 10 July 2019; Published: 12 July 2019 Abstract: Aging is accompanied by widespread changes in brain tissue. Here, we hypothesized that head tissue opacity to near-infrared light provides information about the health status of the brain’s cortical mantle. In diffusive media such as the head, opacity is quantified through the Effective Attenuation Coefficient (EAC), which is proportional to the geometric mean of the absorption and reduced scattering coefficients. EAC is estimated by the slope of the relationship between source–detector distance and the logarithm of the amount of light reaching the detector (optical density).