Chapter 30: Quantum Physics ( ) ( )( ( )( ) ( )( ( )( )
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

7. Gamma and X-Ray Interactions in Matter
Photon interactions in matter Gamma- and X-Ray • Compton effect • Photoelectric effect Interactions in Matter • Pair production • Rayleigh (coherent) scattering Chapter 7 • Photonuclear interactions F.A. Attix, Introduction to Radiological Kinematics Physics and Radiation Dosimetry Interaction cross sections Energy-transfer cross sections Mass attenuation coefficients 1 2 Compton interaction A.H. Compton • Inelastic photon scattering by an electron • Arthur Holly Compton (September 10, 1892 – March 15, 1962) • Main assumption: the electron struck by the • Received Nobel prize in physics 1927 for incoming photon is unbound and stationary his discovery of the Compton effect – The largest contribution from binding is under • Was a key figure in the Manhattan Project, condition of high Z, low energy and creation of first nuclear reactor, which went critical in December 1942 – Under these conditions photoelectric effect is dominant Born and buried in • Consider two aspects: kinematics and cross Wooster, OH http://en.wikipedia.org/wiki/Arthur_Compton sections http://www.findagrave.com/cgi-bin/fg.cgi?page=gr&GRid=22551 3 4 Compton interaction: Kinematics Compton interaction: Kinematics • An earlier theory of -ray scattering by Thomson, based on observations only at low energies, predicted that the scattered photon should always have the same energy as the incident one, regardless of h or • The failure of the Thomson theory to describe high-energy photon scattering necessitated the • Inelastic collision • After the collision the electron departs -

Photon Cross Sections, Attenuation Coefficients, and Energy Absorption Coefficients from 10 Kev to 100 Gev*
1 of Stanaaros National Bureau Mmin. Bids- r'' Library. Ml gEP 2 5 1969 NSRDS-NBS 29 . A111D1 ^67174 tioton Cross Sections, i NBS Attenuation Coefficients, and & TECH RTC. 1 NATL INST OF STANDARDS _nergy Absorption Coefficients From 10 keV to 100 GeV U.S. DEPARTMENT OF COMMERCE NATIONAL BUREAU OF STANDARDS T X J ". j NATIONAL BUREAU OF STANDARDS 1 The National Bureau of Standards was established by an act of Congress March 3, 1901. Today, in addition to serving as the Nation’s central measurement laboratory, the Bureau is a principal focal point in the Federal Government for assuring maximum application of the physical and engineering sciences to the advancement of technology in industry and commerce. To this end the Bureau conducts research and provides central national services in four broad program areas. These are: (1) basic measurements and standards, (2) materials measurements and standards, (3) technological measurements and standards, and (4) transfer of technology. The Bureau comprises the Institute for Basic Standards, the Institute for Materials Research, the Institute for Applied Technology, the Center for Radiation Research, the Center for Computer Sciences and Technology, and the Office for Information Programs. THE INSTITUTE FOR BASIC STANDARDS provides the central basis within the United States of a complete and consistent system of physical measurement; coordinates that system with measurement systems of other nations; and furnishes essential services leading to accurate and uniform physical measurements throughout the Nation’s scientific community, industry, and com- merce. The Institute consists of an Office of Measurement Services and the following technical divisions: Applied Mathematics—Electricity—Metrology—Mechanics—Heat—Atomic and Molec- ular Physics—Radio Physics -—Radio Engineering -—Time and Frequency -—Astro- physics -—Cryogenics. -

Multidisciplinary Design Project Engineering Dictionary Version 0.0.2
Multidisciplinary Design Project Engineering Dictionary Version 0.0.2 February 15, 2006 . DRAFT Cambridge-MIT Institute Multidisciplinary Design Project This Dictionary/Glossary of Engineering terms has been compiled to compliment the work developed as part of the Multi-disciplinary Design Project (MDP), which is a programme to develop teaching material and kits to aid the running of mechtronics projects in Universities and Schools. The project is being carried out with support from the Cambridge-MIT Institute undergraduate teaching programe. For more information about the project please visit the MDP website at http://www-mdp.eng.cam.ac.uk or contact Dr. Peter Long Prof. Alex Slocum Cambridge University Engineering Department Massachusetts Institute of Technology Trumpington Street, 77 Massachusetts Ave. Cambridge. Cambridge MA 02139-4307 CB2 1PZ. USA e-mail: [email protected] e-mail: [email protected] tel: +44 (0) 1223 332779 tel: +1 617 253 0012 For information about the CMI initiative please see Cambridge-MIT Institute website :- http://www.cambridge-mit.org CMI CMI, University of Cambridge Massachusetts Institute of Technology 10 Miller’s Yard, 77 Massachusetts Ave. Mill Lane, Cambridge MA 02139-4307 Cambridge. CB2 1RQ. USA tel: +44 (0) 1223 327207 tel. +1 617 253 7732 fax: +44 (0) 1223 765891 fax. +1 617 258 8539 . DRAFT 2 CMI-MDP Programme 1 Introduction This dictionary/glossary has not been developed as a definative work but as a useful reference book for engi- neering students to search when looking for the meaning of a word/phrase. It has been compiled from a number of existing glossaries together with a number of local additions. -
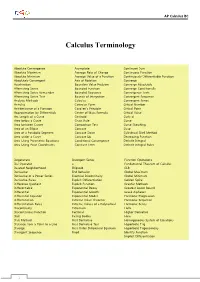
Calculus Terminology
AP Calculus BC Calculus Terminology Absolute Convergence Asymptote Continued Sum Absolute Maximum Average Rate of Change Continuous Function Absolute Minimum Average Value of a Function Continuously Differentiable Function Absolutely Convergent Axis of Rotation Converge Acceleration Boundary Value Problem Converge Absolutely Alternating Series Bounded Function Converge Conditionally Alternating Series Remainder Bounded Sequence Convergence Tests Alternating Series Test Bounds of Integration Convergent Sequence Analytic Methods Calculus Convergent Series Annulus Cartesian Form Critical Number Antiderivative of a Function Cavalieri’s Principle Critical Point Approximation by Differentials Center of Mass Formula Critical Value Arc Length of a Curve Centroid Curly d Area below a Curve Chain Rule Curve Area between Curves Comparison Test Curve Sketching Area of an Ellipse Concave Cusp Area of a Parabolic Segment Concave Down Cylindrical Shell Method Area under a Curve Concave Up Decreasing Function Area Using Parametric Equations Conditional Convergence Definite Integral Area Using Polar Coordinates Constant Term Definite Integral Rules Degenerate Divergent Series Function Operations Del Operator e Fundamental Theorem of Calculus Deleted Neighborhood Ellipsoid GLB Derivative End Behavior Global Maximum Derivative of a Power Series Essential Discontinuity Global Minimum Derivative Rules Explicit Differentiation Golden Spiral Difference Quotient Explicit Function Graphic Methods Differentiable Exponential Decay Greatest Lower Bound Differential -

The Basic Interactions Between Photons and Charged Particles With
Outline Chapter 6 The Basic Interactions between • Photon interactions Photons and Charged Particles – Photoelectric effect – Compton scattering with Matter – Pair productions Radiation Dosimetry I – Coherent scattering • Charged particle interactions – Stopping power and range Text: H.E Johns and J.R. Cunningham, The – Bremsstrahlung interaction th physics of radiology, 4 ed. – Bragg peak http://www.utoledo.edu/med/depts/radther Photon interactions Photoelectric effect • Collision between a photon and an • With energy deposition atom results in ejection of a bound – Photoelectric effect electron – Compton scattering • The photon disappears and is replaced by an electron ejected from the atom • No energy deposition in classical Thomson treatment with kinetic energy KE = hν − Eb – Pair production (above the threshold of 1.02 MeV) • Highest probability if the photon – Photo-nuclear interactions for higher energies energy is just above the binding energy (above 10 MeV) of the electron (absorption edge) • Additional energy may be deposited • Without energy deposition locally by Auger electrons and/or – Coherent scattering Photoelectric mass attenuation coefficients fluorescence photons of lead and soft tissue as a function of photon energy. K and L-absorption edges are shown for lead Thomson scattering Photoelectric effect (classical treatment) • Electron tends to be ejected • Elastic scattering of photon (EM wave) on free electron o at 90 for low energy • Electron is accelerated by EM wave and radiates a wave photons, and approaching • No -

Calculus Formulas and Theorems
Formulas and Theorems for Reference I. Tbigonometric Formulas l. sin2d+c,cis2d:1 sec2d l*cot20:<:sc:20 +.I sin(-d) : -sitt0 t,rs(-//) = t r1sl/ : -tallH 7. sin(A* B) :sitrAcosB*silBcosA 8. : siri A cos B - siu B <:os,;l 9. cos(A+ B) - cos,4cos B - siuA siriB 10. cos(A- B) : cosA cosB + silrA sirrB 11. 2 sirrd t:osd 12. <'os20- coS2(i - siu20 : 2<'os2o - I - 1 - 2sin20 I 13. tan d : <.rft0 (:ost/ I 14. <:ol0 : sirrd tattH 1 15. (:OS I/ 1 16. cscd - ri" 6i /F tl r(. cos[I ^ -el : sitt d \l 18. -01 : COSA 215 216 Formulas and Theorems II. Differentiation Formulas !(r") - trr:"-1 Q,:I' ]tra-fg'+gf' gJ'-,f g' - * (i) ,l' ,I - (tt(.r))9'(.,') ,i;.[tyt.rt) l'' d, \ (sttt rrJ .* ('oqI' .7, tJ, \ . ./ stll lr dr. l('os J { 1a,,,t,:r) - .,' o.t "11'2 1(<,ot.r') - (,.(,2.r' Q:T rl , (sc'c:.r'J: sPl'.r tall 11 ,7, d, - (<:s<t.r,; - (ls(].]'(rot;.r fr("'),t -.'' ,1 - fr(u") o,'ltrc ,l ,, 1 ' tlll ri - (l.t' .f d,^ --: I -iAl'CSllLl'l t!.r' J1 - rz 1(Arcsi' r) : oT Il12 Formulas and Theorems 2I7 III. Integration Formulas 1. ,f "or:artC 2. [\0,-trrlrl *(' .t "r 3. [,' ,t.,: r^x| (' ,I 4. In' a,,: lL , ,' .l 111Q 5. In., a.r: .rhr.r' .r r (' ,l f 6. sirr.r d.r' - ( os.r'-t C ./ 7. /.,,.r' dr : sitr.i'| (' .t 8. tl:r:hr sec,rl+ C or ln Jccrsrl+ C ,f'r^rr f 9. -

Photons Outline
1 Photons Observational Astronomy 2019 Part 1 Prof. S.C. Trager 2 Outline Wavelengths, frequencies, and energies of photons The EM spectrum Fluxes, filters, magnitudes & colors 3 Wavelengths, frequencies, and energies of photons Recall that λν=c, where λ is the wavelength of a photon, ν is its frequency, and c is the speed of light in a vacuum, c=2.997925×1010 cm s–1 The human eye is sensitive to wavelengths from ~3900 Å (1 Å=0.1 nm=10–8 cm=10–10 m) – blue light – to ~7200 Å – red light 4 “Optical” astronomy runs from ~3100 Å (the atmospheric cutoff) to ~1 µm (=1000 nm=10000 Å) Optical astronomers often refer to λ>8000 Å as “near- infrared” (NIR) – because it’s beyond the wavelength sensitivity of most people’s eyes – although NIR typically refers to the wavelength range ~1 µm to ~2.5 µm We’ll come back to this in a minute! 5 The energy of a photon is E=hν, where h=6.626×10–27 erg s is Planck’s constant High-energy (extreme UV, X-ray, γ-ray) astronomers often use eV (electron volt) as an energy unit, where 1 eV=1.602176×10–12 erg 6 Some useful relations: E (erg) 14 ⌫ (Hz) = 1 =2.418 10 E (eV) h (erg s− ) ⇥ ⇥ c hc 1 1 1 λ (A)˚ = = = 12398.4 E− (eV− ) ⌫ ⌫ E (eV) ⇥ Therefore a photon with a wavelength of 10 Å has an energy of ≈1.24 keV 7 If a photon was emitted from a blackbody of temperature T, then the average photon energy is Eav~kT, where k = 1.381×10–16 erg K–1 = 8.617×10–5 eV K–1 is Boltzmann’s constant. -

Electrical Power and Energy
Electric Circuits Name: Electrical Power and Energy Read from Lessons 2 and 3 of the Current Electricity chapter at The Physics Classroom: http://www.physicsclassroom.com/Class/circuits/u9l2d.html http://www.physicsclassroom.com/Class/circuits/u9l3d.html MOP Connection: Electric Circuits: sublevel 3 Review: 1. The electric potential at a given location in a circuit is the amount of _____________ per ___________ at that location. The location of highest potential within a circuit is at the _______ ( +, - ) terminal of the battery. As charge moves through the external circuit from the ______ ( +, - ) to the ______ ( +, - ) terminal, the charge loses potential energy. As charge moves through the battery, it gains potential energy. The difference in electric potential between any two locations within the circuit is known as the electric potential difference; it is sometimes called the _____________________ and represented by the symbol ______. The rate at which charge moves past any point along the circuit is known as the ___________________ and is expressed with the unit ________________. The diagram at the right depicts an electric circuit in a car. The rear defroster is connected to the 12-Volt car battery. Several points are labeled along the circuit. Use this diagram for questions #2-#6. 2. Charge flowing through this circuit possesses 0 J of potential energy at point ___. 3. The overall effect of this circuit is to convert ____ energy into ____ energy. a. electrical, chemical b. chemical, mechanical c. thermal, electrical d. chemical, thermal 4. The potential energy of the charge at point A is ___ the potential energy at B. -

Ionizing Photon Production from Massive Stars
Ionizing Photon Production from Massive Stars Michael W. Topping CASA, Department of Astrophysical & Planetary Sciences, University of Colorado, Boulder, CO 80309 [email protected] ABSTRACT Ionizing photons are important ingredients in our understanding of stellar and interstellar processes, but modeling their properties can be difficult. This paper examines methods of modeling Lyman Continuum (LyC) photon production us- ing stellar evolutionary tracks coupled to a grid of model atmospheres. In the past, many stellar models have not been self-consistent and have not been able to accurately reproduce stellar population spectra and ionizing photon luminosities. We examine new models of rotating stars at both solar and sub-solar metallicity and show that they increase accuracy of ionizing photon production and satisfy self-consistency relationships. Thesis Advisor: Dr. Michael Shull | Department of Astrophysical and Planetary Sciences Thesis Committee: Dr. John Stocke | Department of Astrophysical and Planetary Sciences Dr. William Ford | Department of Physics Dr. Emily Levesque | Department of Astrophysical and Planetary Sciences Defense Date: March 17, 2014 { 2 { Contents 1 INTRODUCTION 3 1.1 Background . .4 1.1.1 Evolutionary Tracks . .4 1.1.2 Model Atmospheres . .5 2 METHODS 6 2.1 EVOLUTIONARY TRACKS . .7 2.1.1 Old Non-Rotating Tracks (Schaller 1992) . .8 2.1.2 New Non-Rotating Tracks . .8 2.1.3 New Rotating Tracks . .8 2.2 Model Atmospheres . 12 2.2.1 Program Setup . 12 2.2.2 Initial Parameter Files . 12 2.2.3 Model Atmosphere Calculation . 13 2.2.4 Defining the Grid . 15 2.3 Putting It All Together . 15 3 RESULTS 20 3.1 Lifetime Integrated H I Photon Luminosities . -

Chapter 4 Light and Matter-Waves And/Or Particles
Chapter 4 Light and Matter-Waves and/or Particles Chapters 4 through 7, taken in sequence, aim to develop an elementary understanding of quantum chemistry: from its experimental and conceptual foundations (Chapter 4), to the one-electron atom (Chapter 5), to the many-electron atom (Chapter 6), andfinally to the molecule (Chapter 7). The present chapter builds to a point where the wave function and uncertainty principle emerge-heuristically, at least-as a way to reconcile the dualistic behavior of waves and particles. Electromagnetic waves undergo interference; so do electrons. “Pinging” atoms transfer energy and momentum; so do photons. A conjned wave develops standing modes; so does the electron in hydrogen. The hope is to instill, above all, a respectfor the manifold difference between a deterministic macroworld and a statistical microworld. The exercises have a further unashamedly practical goal: to help a reader become adept at handling some of the fundamental (yet mathematically simple) equations that underpin quantum mechanics. 1. The relationship connecting the wavelength, frequency, and speed of a wave (especially as applied to electromagnetic radiation): /z v = c 2. The arithmetic of interference, and the double-slit diffraction equation as a corollary: d sin6 = nil 3. The Planck-Einstein formula: E = h v 4. The momentum of a photon: p = i 87 I- . 90 Complete Solutions 3. A wave crest appears only once per cycle. To observe 7200 crests at one point in space is therefore to observe 7200 completed cycles. (a) Convert hours into seconds: 7200 cycles 1 h 1 min v= h x -60 min x -60 s = 2.0 cycles s-’ = 2.0 Hz Two cycles, on the average, are completed every second; thefrequency is 2.0 Hz. -

Photon Energy Upconversion Through Thermal Radiation with the Power Efficiency Reaching 16%
ARTICLE Received 30 May 2014 | Accepted 27 Oct 2014 | Published 28 Nov 2014 DOI: 10.1038/ncomms6669 Photon energy upconversion through thermal radiation with the power efficiency reaching 16% Junxin Wang1,*, Tian Ming1,*,w, Zhao Jin1, Jianfang Wang1, Ling-Dong Sun2 & Chun-Hua Yan2 The efficiency of many solar energy conversion technologies is limited by their poor response to low-energy solar photons. One way for overcoming this limitation is to develop materials and methods that can efficiently convert low-energy photons into high-energy ones. Here we show that thermal radiation is an attractive route for photon energy upconversion, with efficiencies higher than those of state-of-the-art energy transfer upconversion under continuous wave laser excitation. A maximal power upconversion efficiency of 16% is 3 þ achieved on Yb -doped ZrO2. By examining various oxide samples doped with lanthanide or transition metal ions, we draw guidelines that materials with high melting points, low thermal conductivities and strong absorption to infrared light deliver high upconversion efficiencies. The feasibility of our upconversion approach is further demonstrated under concentrated sunlight excitation and continuous wave 976-nm laser excitation, where the upconverted white light is absorbed by Si solar cells to generate electricity and drive optical and electrical devices. 1 Department of Physics, The Chinese University of Hong Kong, Shatin, Hong Kong SAR, China. 2 State Key Laboratory of Rare Earth Materials Chemistry and Applications, Peking University, Beijing 100871, China. * These authors contributed equally to this work. w Present address: Research Laboratory of Electronics, Massachusetts Institute of Technology, Cambridge, Massachusetts 02139, USA. -

Best Glide Speed and Distance
General Aviation FAA Joint Steering Committee Aviation Safety Safety Enhancement Topic Best Glide Speed and Distance The General Aviation Joint Steering Committee (GAJSC) has determined that a significant number of general aviation fatalities could be avoided if pilots were better informed and trained in determining and flying their aircraft at the best glide speed while maneuvering to complete a forced landing. What is Best Glide Speed? Keep in mind that this speed will increase with weight so most manufacturers will establish Is it the speed that will get you the greatest the best glide speed at gross weight for the aircraft. distance? Or is it the speed that gets you the That means your best glide speed will be a little longest time in the air? Or are these two the same lower for lower aircraft weights. — the longer you fly, the further you go? Well, as so often is the case, best glide speed depends on what Need More Time? you’re trying to do. If you’re more interested in staying in the air Going the Distance as long as possible to either fix the problem or to communicate your intentions and prepare for a If it’s distance you want, than you’ll need to forced landing, then minimum sink speed is what use the speed and configuration that will get you you’ll need. This speed is rarely found in Pilot the most distance forward for each increment of Operating Handbooks, but it will be a little slower altitude lost. This is often referred to as best glide than maximum glide range speed.