Introduction to Congenital Heart Disease
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Fetal Blood Flow and Genetic Mutations in Conotruncal Congenital Heart Disease
Journal of Cardiovascular Development and Disease Review Fetal Blood Flow and Genetic Mutations in Conotruncal Congenital Heart Disease Laura A. Dyer 1 and Sandra Rugonyi 2,* 1 Department of Biology, University of Portland, Portland, OR 97203, USA; [email protected] 2 Department of Biomedical Engineering, Oregon Health & Science University, Portland, OR 97239, USA * Correspondence: [email protected] Abstract: In congenital heart disease, the presence of structural defects affects blood flow in the heart and circulation. However, because the fetal circulation bypasses the lungs, fetuses with cyanotic heart defects can survive in utero but need prompt intervention to survive after birth. Tetralogy of Fallot and persistent truncus arteriosus are two of the most significant conotruncal heart defects. In both defects, blood access to the lungs is restricted or non-existent, and babies with these critical conditions need intervention right after birth. While there are known genetic mutations that lead to these critical heart defects, early perturbations in blood flow can independently lead to critical heart defects. In this paper, we start by comparing the fetal circulation with the neonatal and adult circulation, and reviewing how altered fetal blood flow can be used as a diagnostic tool to plan interventions. We then look at known factors that lead to tetralogy of Fallot and persistent truncus arteriosus: namely early perturbations in blood flow and mutations within VEGF-related pathways. The interplay between physical and genetic factors means that any one alteration can cause significant disruptions during development and underscore our need to better understand the effects of both blood flow and flow-responsive genes. -

Patent Ductus Arteriosus About This Factsheet the Normal Heart
Understanding your child’s heart Patent ductus arteriosus About this factsheet The normal heart This factsheet is for parents of babies and children who The heart is a muscular pump which pumps blood through the have patent ductus arteriosus (PDA), which is also known as body and lungs. There are four chambers in the heart. The two persistent arterial duct. upper ones are called the right atrium and left atrium. These are separated by a wall called the atrial septum. The two lower It explains: chambers are called the right and left ventricles, and are separated • what patent ductus arteriosus is and how it is diagnosed by a wall called the ventricular septum. • how patent ductus arteriosus is treated • the benefits and risks of treatments. On each side of the heart, blood passes from the atrium, through a heart valve – the tricuspid valve on the right, and the mitral valve This factsheet does not replace the advice that doctors or on the left – into the ventricle. The ventricles are the main pumping nurses may give you, but it should help you to understand chambers of the heart. Each ventricle pumps blood out into an artery. what they tell you. The right ventricle pumps blood – blue in the illustration – into the pulmonary artery (the blood vessel that takes blood to the lungs). The left ventricle pumps blood – red in the illustration – into the aorta (the blood vessel that takes blood to the rest of the body). Blood flows from the right side of the heart, through the pulmonary valve into the pulmonary artery, and then to the lungs where it picks up oxygen. -

Development of HEART 4-VEINS
Development of brachiocephalic veins 1. Right brachiocephalic vein is formed by cranial part of right anterior cardinal vein and 2. Left brachiocephalic is formed by cranial part of left anterior cardinal vein and the interant.cardinal anastomosis. Development of superior vena cava 1. The part up to the opening of vena azygos develops from caudal part of right ant.cardinal vein and 2. The part below the opening (intrapericardial part) is formed by the right common cardinal vein. Development of azygos and hemiazygos veins A. 1. Vena azygos develops from right azygos line vein and 2. The arch of vena azygos is formed by the cranial end of right postcardinal vein. B. Hemiazygos veins are formed by the left azygos line vein. Development of Inferior vena cava Inferior vena cava is formed, from below upwards by: 1. Begins by the union of the two common iliac veins (postcardinal veins), 2. Right supracardinal, 3. Right supra-subcardinal anastomosis, 4. Right subcardinal, 5. New formation (hepatic segment) and 6. Hepatocardiac channel (terminal part of right vitelline vein). Congenital anomalies • Double inferior vena cava • Absence • Left SVC • Double SVC DEVELOPMENT OF PORTAL VEIN 1. The portal vein is formed behind the neck of pancreas by the union of superior mesentric and splenic vein to the left vitelline vein. 2. The part of the portal vein which is behind the Ist part of duodenum is formed by middle dorsal transverse anastomosis. 3. Part of portal vein which is in the free margin of lesser omentum is formed by cranial or distal part of right vitelline vein. -

Fetal Circulation
The Fetal Circulation Dr. S. Mathieu, Specialist Registrar in Anaesthesia Dr. D. J. Dalgleish, Consultant Anaesthetist Royal Bournemouth and Christchurch Hospitals Trust, UK Questions 1. In the fetal circulation: a) There are two umbilical arteries and one umbilical vein? b) Over 90% of blood passes the liver via the ductus venosus c) The foramen ovale divides the left and right ventricle d) The umbilical artery carries oxygenated blood from the placenta to the fetus e) The foramen ovale allows oxygenated blood to bypass the pulmonary circulation 2. In the fetal circulation: a) The oxygen dissociation curve of fetal haemoglobin is shifted to the left compared with adult haemoglobin ensuring oxygen delivery to the fetus despite low oxygen partial pressures b) It is the presence of the ductus arteriosus and large pulmonary vascular resistance which ensures most of the right ventricular output passes into the aorta c) The patency of the ductus arteriosus is maintained by high oxygen tensions d) The patency of the ductus arteriosus is maintained by the vasodilating effects of prostaglandin G2 e) 2,3-DPG levels are higher in fetal haemoglobin compared with adult haemaglobin 3. Changes at birth include: a) a fall in pulmonary vascular resistance b) a rise in systemic vascular resistance with clamping of the cord c) an increase in hypoxic pulmonary vasoconstriction d) a rise in left atrial pressure e) closure of the ductus arteriosus within 24 hours 4. The following congenital heart lesions are cyanotic: a) Ventricular septal defect b) Atrial septal defect c) Patent ductus arteriosus d) Tetralogy of Fallot e) Transposition of the great arteries MCQ answers at end Key points • The fetal circulation supplies the fetal tissues with oxygen and nutrients from the placenta. -

The Pattern and Mechanisms of Response to Oxygen by the Ductus Arteriosus and Umbilical Artery
Pediat. Res. 6: 693-700 (1972) Acetylcholine neonate atropine prematurity bradykinin sympathetic nervous system ductus arteriosus The Pattern and Mechanisms of Response to Oxygen by the Ductus Arteriosus and Umbilical Artery INGRID OBERHANSLI-WEISS, MICHAEL A. HEYMANN1391, ABRAHAM M. RUDOLPH, AND KENNETH L. MELMON Cardiovascular Research Institute and Departments of Pediatrics, Physiology and Pharmacology, University of California San Francisco, San Francisco, California, USA Extract Response of the ductus arteriosus and umbilical artery to changes in oxygen tension, to acetylcholine, and to sympathetic and parasympathetic blocking agents was studied in vitro in isolated rings obtained from 22 fetal lambs of 98- to 147-day gestation. After stabilization of tension at a baseline level (0.3-0.7 g) in a PO2 environment of 35-45 mm Hg, both increase of the PO2 to 550 mm Hg and decrease of the PO2 to 8 mm Hg of the bathing solution produced constriction. The mean maximal tension developed by the ductus arteriosus.was 3.91 g at high PO2 and 3.87 g at low POr The increase in maximal tension developed with advancing gestation was also similar at both high and low POj. At P02 levels of 8-550 mm Hg, acetylcholine produced a further increase in tension, whereas bradykinin only produced an increase in tension at high PO2- Alpha and beta sympathetic blockade had no effect on the constrictor response to oxygen. Atropine relaxed the ductus arteriosus and umbilical artery at both high and low Po2 levels; the degree of relaxation was related to drug concentration. Acetylcholin- esterase also relaxed the ductus arteriosus constricted by oxygen. -

Echocardiographic Follow-Up of Patent Foramen Ovale and the Factors Affecting Spontaneous Closure
Acta Cardiol Sin 2016;32:731-737 Brief Report doi: 10.6515/ACS20160205A Echocardiographic Follow-Up of Patent Foramen Ovale and the Factors Affecting Spontaneous Closure Ali Yildirim,1 Alperen Aydin,2 Tevfik Demir,1 Fatma Aydin,2 Birsen Ucar1 and Zubeyir Kilic1 Background: The aim of the present study was to evaluate the echocardiographic follow-up of patent foramen ovale, which is considered a potential etiological factor in various diseases, and to determine the factors affecting spontaneous closure. Methods: Between January 2000 and June 2012, records of 918 patients with patent foramen ovale were retrospectively reviewed. Patency of less than 3 mm around the fossa ovalis is called patent foramen ovale. Patients with cyanotic congenital heart diseases, severe heart valve disorders and severe hemodynamic left to right shunts were excluded from the study. The patients were divided into three groups based on age; 1 day-1 monthingroup1,1month-12monthsingroup2,andmorethan12monthsingroup3. Results: Of the 918 patients, 564 (61.4%) had spontaneous closure, 328 (35.8%) had patent foramen ovale continued, 15 (1.6%) patients had patent foramen ovale enlarged to 3-5 mm, 6 patients were enlarged to 5-8 mm, and in one patient patent foramen ovale reached to more than 8 mm size. Defect was spontaneously closed in 65.9% of the patients in group 1, 66.7% of the patients in group 2, and 52.3% of the patients in group 3. There was a negative correlation between the age of diagnosis and spontaneous closure (p < 0.05). Gender, prematurity and coexisting malformations such as patent ductus arteriosus and atrial septal aneurysm did not have any effect on spontaneous closure of patent foramen ovale (p > 0.05). -

Cardiovascular System Note: the Cardiovascular System Develops Early (Week 3), Enabling the Embryo to Grow Beyond the Short
Lymphatics: Lymph vessel formation is similar to blood angiogenesis. Lymphatics begin as lymph sacs in three regions: jugular (near brachiocephalic veins); cranial abdominal (future cysterna chyla); and iliac region. Lym- phatic vessels (ducts) form as outgrowths of the sacs. mesenchyme Lymph nodes are produced by localized mesoder- sinusoid lymph duct lumen mal invaginations that partition the vessel lumen into sinu- soids. The mesoderm develops a reticular framework within which mesodermal lymphocytes accumulate. The spleen and hemal nodes (in ruminants) invagination develop similar to the way lymph nodes develop. Lymph Node Formation Prior to birth, fetal circulation is designed for an in utero aqueous environment where the pla- centa oxygenates fetal blood. Suddenly, at birth... Three In-Utero Adjustments ductus Stretching and constriction of arteriosus umbilical arteries shifts fetal blood flow aortic arch from the placenta to the fetus. Reduced pulmonary trunk L atrium venous return through the (left) umbili- foramen ovale R cal vein and ductus venosus allows the atrium latter to gradually close (over a period caudal vena cava of days). Bradykinin released by expand- ductus venosus ing lungs and increased oxygen concen- tration in blood triggers constriction of aorta the ductus arteriosus which, over two liver months, is gradually converted to a fibrous structure, the ligamentum arte- umbilical v. riosum. portal v. The increased blood flow to the lungs and then to the left atrium equalizes pres- sure in the two atria, resulting in closure umbilical aa. of the foramen ovale that eventually grows permanent. 29 The cardiogenic area, the place where the embryonic heart originates, is located . -

6 Development of the Great Vessels and Conduction Tissue
Development of the Great Vessels and Conduc6on Tissue Development of the heart fields • h:p://php.med.unsw.edu.au/embryology/ index.php?6tle=Advanced_-_Heart_Fields ! 2 Septa6on of the Bulbus Cordis Bulbus Cordis AV Canal Ventricle Looking at a sagital sec6on of the heart early in development the bulbus cordis is con6nuous with the ventricle which is con6nuous with the atria. As the AV canal shiOs to the right the bulbus move to the right as well. Septa6on of the Bulbus Cordis A P A P The next three slides make the point via cross sec6ons that the aorta and pulmonary arteries rotate around each other. This means the septum between them changes posi6on from superior to inferior as well. Septa6on of the Bulbus Cordis P A A P Septa6on of the Bulbus Cordis P A P A Migra6on of neural crest cells Neural crest cells migrate from the 3ed, 4th and 6th pharyngeal arches to form some of the popula6on of cells forming the aor6copulmonary septum. Septa6on of the Bulbus Cordis Truncal (Conal) Swellings Bulbus Cordis The cardiac jelly in the region of the truncus and conus adds the neural crest cells and expands as truncal swellings. Septa6on of the Bulbus Cordis Aorticopulmonary septum These swellings grow toward each other to meet and form the septum between the aorta and pulmonary artery. Aorta Pulmonary Artery Septa6on of the Bulbus Cordis Anterior 1 2 3 1 2 3 The aor6copulmonary septum then rotates as it moves inferiorly. However, the exact mechanism for that rota6on remains unclear. Septa6on of the Bulbus Cordis Aorta Pulmonary Artery Conal Ridges IV Foramen Membranous Muscular IV Endocarial Septum Interventricular Cushion Septum However, the aor6copulmonary septum must form properly for the IV septum to be completed. -

Characterization of the Placenta in the Newborn with Congenital Heart Disease: Distinctions Based on Type of Cardiac Malformation
Pediatric Cardiology https://doi.org/10.1007/s00246-018-1876-x ORIGINAL ARTICLE Characterization of the Placenta in the Newborn with Congenital Heart Disease: Distinctions Based on Type of Cardiac Malformation Jack Rychik1,2,10 · Donna Goff3 · Eileen McKay4 · Antonio Mott5 · Zhiyun Tian1,2 · Daniel J. Licht6,7 · J. William Gaynor8,9 Received: 15 January 2018 / Accepted: 3 April 2018 © The Author(s) 2018 Abstract The placenta is a complex organ that influences prenatal growth and development, and through fetal programming impacts postnatal health and well-being lifelong. Little information exists on placental pathology in the presence of congenital heart disease (CHD). Our objective is to characterize the placenta in CHD and investigate for distinctions based on type of mal- formation present. Placental pathology from singleton neonates prenatally diagnosed and delivered at > 37 weeks gestation was analyzed. Placental findings of absolute weight, placental weight-to-newborn birth weight ratio, chorangiosis, villus maturity, thrombosis, and infarction were recorded and analyzed based on four physiological categories of CHD: (1) single ventricle-aortic obstruction, (2) single ventricle-pulmonic obstruction, (3) two-ventricle anomalies, and (4) transposition of the great arteries (TGA). Associations between fetal Doppler assessments of middle cerebral/umbilical arterial flow and placental findings were investigated. A total of 120 cases of complex CHD were analyzed. Overall placental-to-birth weight ratios were < 10th percentile for 77% and < 3rd percentile for 49% with abnormalities of chorangiosis (18%), hypomature villi (15%), thrombosis (41%), and infarction (17%) common. There was no association between fetal Doppler flow measures and placental abnormalities. Newborns with TGA had the greatest degree of placental abnormality. -
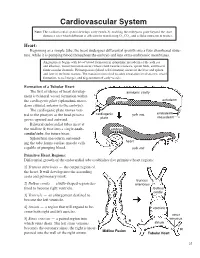
Cardiovascular System Note: the Cardiovascular System Develops Early (Week-3), Enabling the Embryo to Grow Beyond the Short
Cardiovascular System Note: The cardiovascular system develops early (week-3), enabling the embryo to grow beyond the short distances over which diffusion is efficient for transferring 2O , CO2, and cellular nutrients & wastes. Heart: Beginning as a simple tube, the heart undergoes differential growth into a four chambered struc- ture, while it is pumping blood throughout the embryo and into extra-embryonic membranes. Angiogenesis begins with blood island formation in splanchnic mesoderm of the yolk sac and allantois. Vessel formation occurs when island vesicles coalesce, sprout buds, and fuse to form vascular channels. Hematopoiesis (blood cell formation) occurs in the liver and spleen and later in the bone marrow. The transition from fetal to adult circulation involves new vessel formation, vessel merger, and degeneration of early vessels. Formation of a Tubular Heart: The first evidence of heart develop- amnionic cavity ment is bilateral vessel formation within ectoderm the cardiogenic plate (splanchnic meso- embryo derm situated anterior to the embryo). The cardiogenic plate moves ven- tral to the pharynx as the head process cardiogenic yolk sac endoderm mesoderm grows upward and outward. plate Bilateral endocardial tubes meet at the midline & fuse into a single endo- embryo cardial tube, the future heart. Splanchnic mesoderm surround- ing the tube forms cardiac muscle cells heart capable of pumping blood. yolk sac Primitive Heart Regions: Differential growth of the endocardial tube establishes five primitive heart regions: 1] Truncus arteriosus — the output region of the heart. It will develop into the ascending aorta and pulmonary trunk. truncus 2] Bulbus cordis — a bulb-shaped region des- arteriosus tined to become right ventricle. -

[email protected]
Embryology)**@gmail.com 1-To serve prenatal needs. 2-To permit modifications at birth, which establish the neonatal circulation #Remember : Good respiration in the newborn infant is dependent upon normal circulatory changes at birth. Three structures are very important in the transitional circulation: 1- Ductus venosus. 2- Ductus arteriosus. 3- Foramen ovale Embryology)**@gmail.com Blood reaches & leaves the fetus through the umbilical cord and it Contains two arteries and one vein. 1- Highly oxygenated blood passes from the placenta through the umbilical vein. 2-Half of this blood reaches the IVC through the ductus venosus. 3- The other half passes to liver sinusoids then to the IVC. 4- Blood of the IVC reaches the right atrium, then left atrium through the Foramen Ovale (an opening between the two atrium). 5- Then to the left ventricle to the ascending aorta, and the aortic arch to supply head & neck brain, cardiac muscle and upper limbs with highly oxygenated blood. 6- Small amount of highly oxygenated blood in right atrium mixes with venous blood of the SVC passes to right ventricle. 7- Then to the pulmonary artery (lungs are not functioning yet) then to ductus arteriosus to the descending aorta, to lower half of fetal body. 8- Then back to placenta via the two umbilical arteries. Embryology)**@gmail.com 12 # After Ligation of the umbilical cord there will be Sudden fall of blood pressure in the IVC and the right Atrium Also the valve of the ductus venosus constricts. # After Aeration ( ventilation ) of the lungs at birth: 1- Marked increase in the pulmonary blood flow due to functioning of the lungs and increase pressure in left atrium causing physiological colsure. -

Truncus Arteriosus What the Nurse Caring for the Patient with Congenital Heart Disease Needs to Know
Truncus Arteriosus What the Nurse Caring for the Patient with Congenital Heart Disease Needs to Know Mary Rummell, MN, RN, CPNP, CNS, FAHA Clinical Nurse Specialist, Pediatric Cardiology, Cardiac Services, Oregon Health & Science University (Retired) Embryology Rare congenital heart disease Before the 4th week of embryonic life o Endocardial tube expands, elongates and develops areas of dilation . Includes bulbus cordis . Ventricular outflow tracks . Truncus arteriosus o Results in cardiac looping – completed by day 28 of gestation . Stretching creates torsion in truncus arteriosus . Contributes to formation of spiral septum Septation of the truncus arteriosus – day 26 to 42 o Mesenchymal cells . Include neural crest cells . Actively proliferate . Form ridges in bulbus cordis and truncus arteriosus . Ridges create 180 degree spiraling . Create aorticopulmonary septum . Contribute to closure of conal ventricular septum o Spiraling enhanced by forward blood flow o Failure of septation results in: . Truncus arteriosus (TA) . Large ventricular septal defect (VSD) Development of semilunar valves o At base of the truncus arteriosus . Result from: Swelling of endocardial tissue Endocardial cushions o Failure of septation . Results in one valve . Valve abnormal Usually have more than 3 cusps Cusps usually thickened and deformed May be regurgitant Sometimes stenotic Neural crest cells o Arise from genetic material o Influence the development of thymus and parathyroid glands from the pharyngeal pouches 1 o Result in increased prevalence