The Cystinosis Advocate
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Share Your Insight at Komen.Org
frontThe Susan G. Komen Breast Cancer Foundation’sline Newsletter First Quarter 2004 Share Your Insight at Komen.org omen.org has always been a trusted source of breast cancer and Do you think much progress has been made Kbreast health information, but now it also features the world’s first in the fight against breast cancer? online polling center dedicated solely to breast cancer. In conjunction with World Cancer Day on February 4, the Komen Foundation launched Share Your Insight, an online polling campaign that encourages women and men around the globe to voice their opinions about critical breast cancer issues. The Komen Foundation’s newly redesigned Web site, www.komen.org, features monthly polls that will improve our understanding of issues that are important to key audiences, enabling the Foundation to better serve its constituency in its mission to put an end to breast cancer. Help us spread the word about the Share Your Insight campaign by mailing the postcard we’ve included in this issue of Frontline to a friend. “As a patient advocacy organization, what better way for the Komen Foundation to understand the needs of breast cancer patients and their families and represent their views on breast cancer issues than to ask them directly?” said Susan Braun, president and chief executive officer of the Komen Foundation. “Share Your Insight is a real-time forum that allows patients, their families and anyone involved in the fight against breast cancer to tell us what they think about issues relevant to them and to our mission.” Visit www.komen.org to take the poll and have the option to join the Komen Foundation’s polling panel. -

Cause-Related Marketing: a Critical Look at Marketplace Meeting
Running head: CAUSE-RELATED MARKETING 1 Cause-related Marketing A Critical Look at Marketplace Meeting Philanthropy Lindsey Winneroski A Senior Thesis submitted in partial fulfillment of the requirements for graduation in the Honors Program Liberty University Spring 2015 CAUSE-RELATED MARKETING 2 Acceptance of Senior Honors Thesis This Senior Honors Thesis is accepted in partial fulfillment of the requirements for graduation from the Honors Program of Liberty University. ______________________________ Lynnda S. Beavers, Ph.D. Thesis Chair ______________________________ George Young, Ph.D. Committee Member ______________________________ Amy Bonebright, M.A. Committee Member ______________________________ Brenda Ayres, Ph.D. Honors Director ______________________________ Date CAUSE-RELATED MARKETING 3 Abstract The following discussion offers a critical look at cause-related marketing (CRM), a strategic partnership between a corporate and nonprofit entity in which a portion of product sales, or a one-time donation, is given in support of a cause. CRM is an extension of a corporation’s social responsibility efforts in a push to meet increasing consumer demand for organizational accountability and social-consciousness. The discussion examines factors that have fed the mandate for corporate social responsibility, including a connection through online platforms and a generational cohort with a demand to “give back.” Research shows benefits of implementing CRM; however, many ethical issues must be considered when organizations attempt to blend for-profit motives with altruism. CRM and its impact on the definition of philanthropy will be evaluated through the investigation of two campaigns—the Susan G. Komen Pink Campaign and the ALS ice bucket challenge. CAUSE-RELATED MARKETING 4 Cause-related Marketing A Critical Look at Marketplace Meeting Philanthropy In today’s marketplace, consumers expect businesses not only to meet individuals’ needs through products or services but also to be socially responsible by giving back to society. -
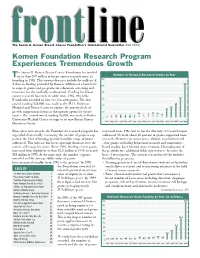
Komen Foundation Research Program Experiences Tremendous Growth
frontThe Susan G. Komen Breast Cancer Foundation’s lineInternational Newsletter Fall 2000 Komen Foundation Research Program Experiences Tremendous Growth he Susan G. Komen Breast Cancer Foundation has funded Number of National Research Grants by Year T more than $47 million in breast cancer research since its founding in 1982. This amount does not include the millions of 120 102 dollars in funding provided by Komen Affiliates at a local level 100 to support grants and programs for education, screening and treatment for the medically underserved. Funding for breast 80 cancer research has risen steadily since 1982, when the Foundation awarded its first two research grants. The first 60 59 award, totaling $28,000, was made to the M.D. Anderson 40 40 31 Hospital and Tumor Center to explore the potential role of 24 20 growth suppression factors as therapeutic agents for breast 12 11 9 11 5 7 5 7 7 8 cancer. The second award, totaling $2,000, was made to Baylor 2 3 4 University Medical Center to support its new Breast Cancer 0 1982 1983 1984 1985 19861987 1988 1989 1990 1991 1992 1993 1994 1995 1996 1997 1998 1999 Detection Center. Since these first awards, the Foundation’s research program has increased since 1982, but so has the diversity of research topics expanded dramatically, increasing the number of projects sup- addressed. Overall, about 65 percent of grants supported basic ported, the level of funding provided and the scope of issues research. However, in recent years, clinical, translational and addressed. This increase has been especially dramatic over the other grants, including behavioral research and community- course of the past five years. -

The Concordia Summit Announces Second Annual Forum
FOR IMMEDIATE RELEASE The Concordia Summit Announces Second Annual Forum The Concordia Summit Announces Second Annual Forum Featuring Keynote Address From Former President Bill Clinton and Launch of Partnership With US-Afghan Women's Council Concordia is a Singular Convening of Former and Current Heads of State, Business Executives and Non-Profit Leaders Devoted to Promoting Public-Private Partnerships. Bill Clinton to be joined by Laura Bush, John McCain, Donna Karan, Alvaro Uribe, Thomas Kean, Mikheil Saakashvili, and more NEW YORK, Sept. 13, 2012 The Advisory Board and Co-founders of The Concordia Summit (Concordia) today announced that the 2nd Annual Concordia Summit will be held on September 27, 2012 in New York at the Plaza Hotel. The summit will feature a keynote address by former U.S. President William J. Clinton. The Concordia Summit is a non-profit organization dedicated to promoting public-private partnerships around pressing global issues. Concordia also announced a new partnership with the U.S.-Afghan Women's Council (USAWC) that will include the development of a 10-year strategic plan that will further ensure public and private investment in children's and women's initiatives in Afghanistan. In addition to President Clinton, participants of this year's Concordia Summit will include United States Senator from Arizona, John McCain; founder of the Susan G. Komen for the Cure breast cancer organization, Nancy Brinker; former First Lady Laura Bush (appearing via video); The Deputy Prime Minister of Ireland and Organization for Security and Co-operation in Europe Chairman-in- Office, Eamon Gilmore; fashion designer Donna Karan; The former Governor of New Jersey, Thomas Kean; the former President of Poland, Aleksander Kwasniewski; former United States Representative from California, Jane Harman; U.S. -

Welcome to the Texas Women's HALL of FAME 2014 PROGRAM
GCW_HOF_program_042514.indd 1 4/28/14 9:20 AM TEXAS Women’s hall of fAME Welcome to The Texas Women’s HALL OF FAME 2014 PROGRAM Welcome Carmen Pagan, Governor’s Commission for Women Chair Invocation Reverend Coby Shorter Presentation The Anita Thigpen Perry School of Nursing at Texas Tech University Keynote Address Governor Rick Perry Induction 2014 Texas Women’s Hall of Fame Honorees Closing 3 Texas Governor‘s Commission for Women GCW_HOF_program_042514.indd 2-3 4/28/14 9:20 AM TEXAS Women’s hall of fAME TEXAS Women’s hall of fAME The Texas Women’s HALL OF FAME AWARDS The Governor’s Commission for Women established the Texas Women’s Hall of Fame in 1984 to honor the remarkable achievements of Texas women while sharing their stories of great determination and innovation. The biennial awards highlight Texas women who have made significant contributions, often despite great odds. Nominations are submitted from across the state and reviewed by a panel of judges. Past honorees include first ladies, Olympic athletes and astronauts. The Texas Women’s HALL OF FAME 2014 Inductees The History of Our HALL OF FAME EXHIBIT In 2003, the Governor’s Commission for Women established a permanent exhibit for the Texas Women’s Hall of Fame on the campus of Texas Woman’s University in Denton, Texas. The exhibit features the biographies, photographs and video interviews of more than 100 notable women who have been chosen to represent the very best from our state. The exhibit is free of charge, and it is open to the public Monday through Friday from 8:00 a.m. -

Gerald R. Ford Oral History Project Nancy Brinker Interviewed by Richard Norton Smith November 19, 2010
Gerald R. Ford Oral History Project Nancy Brinker Interviewed by Richard Norton Smith November 19, 2010 Smith: Thank you very much for doing this. You obviously have a remarkable story in your own right. How did your path cross with that of Betty Ford. Brinker: Well, you know, after my sister died, or as we went through the journey of her very unsuccessful end with breast cancer— Smith: How long was she ill? Brinker: She was diagnosed in 1977 at the end of the year, I think it was, and she died in 1980 at the age of 36. I’ll never forget it. In a lot of ways, it was as if it were yesterday. In fact, I just wrote a book about it, Promise Me, which has a picture of Mrs. Ford in it. So, you know, in those days, cancer was “The Big C,” it was spoken of in whispers. People crossed the streets sometimes when they saw my sister. We lived in Peoria, Illinois and we know there was cancer there, being in hospitals. Still it was nothing like it is today. People were much less willing to address these issues out loud. Smith: Was there even more of the stigma, if that’s the word, attached to breast cancer? Brinker: Yes, of course, even though it was “The Big C,” that was usually lung cancer or maybe an intestinal cancer. But when it came to breast cancer, it was even worse. It was more hidden in the shadows. You couldn’t print the word ‘breast’ in most newspapers or magazines and certainly couldn’t use the words on TV or radio. -

Chief Executive Officer Search
CHIEF EXECUTIVE OFFICER SEARCH 1 Canal Street • PO Box 335 • Seneca Falls, NY 13148 • womenofthehall.org • (315) 568-8060 T HE S EA RC H The Board of Directors of the National Women’s Hall of Fame invites applications and nominations of highly experienced, energetic, and creative candidates for the position of Chief Executive Officer (CEO). Candidates should be attracted to the opportunity to provide highly transformative leadership for the nation’s premier institution honoring exceptional American women who embody the National Women’s Hall of Fame mission of “Showcasing great women . Inspiring all”. The National Women’s Hall of Fame (NWHF/the Hall) is expanding in every way – in size, in reach, in influence. To better accommodate these ambitions, the NWHF rehabilitated the historic 1844 Seneca Knitting Mill located on the Seneca-Cayuga branch of the Erie Canal in Seneca Falls, NY, and moved into it in 2020. This extraordinary achievement was completed over nine years with 10 million dollars of funding. The NWHF is eager to embrace the opportunities enabled by this new, expansive space, including honoring the importance and sense of “place” that Seneca Falls and the Erie Canal system have played in the history of the economic, social, and human rights movements of the United States of America. Following this historic move, in this historic year celebrating the centennial of women’s suffrage, the National Women’s Hall of Fame now seeks a talented, proven leader dedicated to expanding the Hall’s national footprint, advancing its fundraising capacity, strengthening its organizational structure, and planning and implementing an ambitious agenda of new programs and exhibits. -

SURVIVAL QUOTES How to Stay Inspired and Motivated to Succeed During Tough Times LARRY W
LIFE’S LITTLE BOOK OF SURVIVAL QUOTES How to Stay Inspired and Motivated to Succeed During Tough Times LARRY W. POWELL Copyright © 2013 Larry Powell Scribd Edition *** Published by Lifes-Little-Book-Of.com *** This book contains material protected under International and Federal Copyright Laws and Treaties. Any unauthorized reprint or use of this material is prohibited. No part of this book may be reproduced or transmitted in any form or by any means, electronic or mechanical, including photocopying, recording, scanning or by any information storage and retrieval system without express written permission from the author. [email protected] All Rights Reserved. Balloon design by Robert Whiteman from The Noun Project Scribd Edition, License Notes This ebook is licensed for your personal enjoyment only. This ebook may not be re-sold or given away to other people. If you would like to share this book with another person, please purchase an additional copy for each recipient. If you’re reading this book and did not purchase it, or it was not purchased for your use only, then please return to Scribd.com and purchase your own copy. Thank you for respecting the hard work of this author. “I can’t say that I’m all that interested in how people rise and fall. But I am enormously interested in how they fall and rise.” ~Larry Powell Table of Contents PREFACE ............................................................................................................8 INTRODUCTION ..........................................................................................................10 -

Faye Glenn Abdellah 1919 - • As a Nurse Researcher Transformed Nursing Theory, Nursing Care, and Nursing Education
Faye Glenn Abdellah 1919 - • As a nurse researcher transformed nursing theory, nursing care, and nursing education • Moved nursing practice beyond the patient to include care of families and the elderly • First nurse and first woman to serve as Deputy Surgeon General Bella Abzug 1920 – 1998 • As an attorney and legislator championed women’s rights, human rights, equality, peace and social justice • Helped found the National Women’s Political Caucus Abigail Adams 1744 – 1818 • An early feminist who urged her husband, future president John Adams to “Remember the Ladies” and grant them their civil rights • Shaped and shared her husband’s political convictions Jane Addams 1860 – 1935 • Through her efforts in the settlement movement, prodded America to respond to many social ills • Received the Nobel Peace Prize in 1931 Madeleine Korbel Albright 1937 – • First female Secretary of State • Dedicated to policies and institutions to better the world • A sought-after global strategic consultant Tenley Albright 1934 – • First American woman to win a world figure skating championship; triumphed in figure skating after overcoming polio • First winner of figure skating’s triple crown • A surgeon and blood plasma researcher who works to eradicate polio around the world Louisa May Alcott 1832 – 1888 • Prolific author of books for American girls. Most famous book is Little Women • An advocate for abolition and suffrage – the first woman to register to vote in Concord, Massachusetts in 1879 Florence Ellinwood Allen 1884 – 1966 • A pioneer in the legal field with an amazing list of firsts: The first woman elected to a judgeship in the U.S. First woman to sit on a state supreme court. -

SENATE—Wednesday, January 20, 2010
240 CONGRESSIONAL RECORD—SENATE, Vol. 156, Pt. 1 January 20, 2010 SENATE—Wednesday, January 20, 2010 The Senate met at 10 a.m. and was for 1 hour, with Senators allowed to possible to ignore their grief over grow- called to order by the Honorable TOM speak therein for up to 10 minutes ing foreclosures or the uncertainty of UDALL, a Senator from the State of each. The time will be equally divided unemployment or the frustration of New Mexico. and controlled between the two leaders fighting insurance companies for their or their designees. families’ health. PRAYER Following morning business, the Sen- It is just as evident that the people of The Chaplain, Dr. Barry C. Black, of- ate will proceed to executive session to Nevada and the Nation need us to work fered the following prayer: consider the nomination of Beverly toward sensible solutions rather than Let us pray. Baldwin Martin of Georgia to be a U.S. drown once again in the partisan bick- Almighty God, our Heavenly Father, circuit judge for the Eleventh Circuit. ering that consumed much of last year. thank You for the gift of a new year. Debate on the nomination is limited to Some elections go your way; some We have received great benefits from 1 hour, equally divided and controlled elections go the other way. It is the na- Your hands and lift to You our grateful between Senators LEAHY and SESSIONS ture of democratic politics in a very di- praise. or their designees. Upon the use or verse Nation. But regardless of an out- Lord, lead our lawmakers on the road yielding back of the time, the Senate come of an election, as I have said You have chosen. -

1999 Brinker International Awards for Breast Cancer Research Announced
frontThe Susan G. Komen Breast Cancer Foundation’s lineInternational Newsletter Winter 2000 1999 Brinker International Awards for Breast Cancer Research Announced he Komen Foundation presented the 1999 Brinker and makes the award selections. Nomination guidelines are T International Awards for Breast Cancer Research at the available through the International Headquarters of the 22nd Annual San Antonio Breast Cancer Symposium this past Komen Foundation. December. Established by the Foundation in 1992, the two awards recognize leading scientists for significant work that Mary-Claire King, advances basic research concepts or clinical application in the Ph.D., professor of fields of breast cancer research, screening or treatment. medicine and genetics at the University of The 1999 Basic Science Award was presented to Mary-Claire Washington School King, Ph.D., for her work in understanding the role of genetics of Medicine, discov- in breast cancer. The 1999 Clinical Research Award was present- ered that inheriting a ed to Nancy E. Davidson, M.D., for her work with the role of mutation in a single estrogen and steroid receptors in breast cancer growth. Past gene could cause Brinker International Award recipients include such luminaries breast cancer. Until as V. Craig Jordan, Ph.D., D.Sc., and Gabriel Hortobágyi, M.D. Mary-Claire King, Ph.D., and Susan Braun, president and her presentation at CEO of the Foundation. the American Society “I am especially pleased about this year’s Brinker Award of Human Genetics meeting in October of 1990, geneticists recipients,” said Nancy Brinker, founding chair of the Komen thought that it was technically impossible to identify predispos- Foundation. -

Department of State
DEPARTMENT OF STATE 2201 C Street NW., 20520, phone 647–4000 COLIN L. POWELL, Secretary of State; born on April 5, 1937, in New York, NY; education: B.S., City College of New York; M.B.A., George Washington University; military and public service: Army ROTC; participated while attending City College of New York, and received a commission as a 2nd Lieutenant upon graduation; U.S. Army, served 1958– 1993, rising to the rank of General (4 Stars); National Security Adviser for President Ronald Reagan, 1987–1989; Chairman of the Joint Chiefs of Staff, 1989–1993; Chairman, President’s Summit for America’s Future, 1997; Chairman, America’s Promise—The Alliance for Youth, 1997–2000; awards: Presidential Medal of Freedom; Congressional Gold Medal; Presidential Citizens Medal; Secretary of State’s Distinguished Service Medal; author: My American Jour- ney, 1995; family: married to the former Alma Johnson; three children: Michael, Linda, and Anne; nominated by President George W. Bush to become the 65th Secretary of State, and was confirmed by the U.S. Senate on January 20, 2001. OFFICE OF THE SECRETARY Secretary of State.—Colin L. Powell, 647–5291. Executive Assistant.—Craig Kelly, 647–9572. OFFICE OF THE DEPUTY SECRETARY Deputy Secretary of State.—Richard L. Armitage, room 7220, 647–9641. Executive Assistant.—Randall Schriver, (Randy), 647–8931. EXECUTIVE SECRETARIAT Special Assistant and Executive Secretary.—Maura Harty, room 7224, 647–5301. Deputy Executive Secretaries: Daniel B. Smith, 647–6548; Deborah Graze, 647–5302; Carol Rodley, 647–8448. POLICY PLANNING STAFF Director.—Richard Haass, room 7531, 647–2972. Principal Deputy Director.—Donald Steinberg, 647–2372.