A Comparative Case Study of the Susan G. Komen Foundation
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Power Seeking and Backlash Against Female Politicians
Article Personality and Social Psychology Bulletin The Price of Power: Power Seeking and 36(7) 923 –936 © 2010 by the Society for Personality and Social Psychology, Inc Backlash Against Female Politicians Reprints and permission: sagepub.com/journalsPermissions.nav DOI: 10.1177/0146167210371949 http://pspb.sagepub.com Tyler G. Okimoto1 and Victoria L. Brescoll1 Abstract Two experimental studies examined the effect of power-seeking intentions on backlash toward women in political office. It was hypothesized that a female politician’s career progress may be hindered by the belief that she seeks power, as this desire may violate prescribed communal expectations for women and thereby elicit interpersonal penalties. Results suggested that voting preferences for female candidates were negatively influenced by her power-seeking intentions (actual or perceived) but that preferences for male candidates were unaffected by power-seeking intentions. These differential reactions were partly explained by the perceived lack of communality implied by women’s power-seeking intentions, resulting in lower perceived competence and feelings of moral outrage. The presence of moral-emotional reactions suggests that backlash arises from the violation of communal prescriptions rather than normative deviations more generally. These findings illuminate one potential source of gender bias in politics. Keywords gender stereotypes, backlash, power, politics, intention, moral outrage Received June 5, 2009; revision accepted December 2, 2009 Many voters see Senator Hillary Rodham Clinton as coldly politicians and that these penalties may be reflected in voting ambitious, a perception that could ultimately doom her presi- preferences. dential campaign. Peter Nicholas, Los Angeles Times, 2007 Power-Relevant Stereotypes Power seeking may be incongruent with traditional female In 1916, Jeannette Rankin was elected to the Montana seat in gender stereotypes but not male gender stereotypes for a the U.S. -

Share Your Insight at Komen.Org
frontThe Susan G. Komen Breast Cancer Foundation’sline Newsletter First Quarter 2004 Share Your Insight at Komen.org omen.org has always been a trusted source of breast cancer and Do you think much progress has been made Kbreast health information, but now it also features the world’s first in the fight against breast cancer? online polling center dedicated solely to breast cancer. In conjunction with World Cancer Day on February 4, the Komen Foundation launched Share Your Insight, an online polling campaign that encourages women and men around the globe to voice their opinions about critical breast cancer issues. The Komen Foundation’s newly redesigned Web site, www.komen.org, features monthly polls that will improve our understanding of issues that are important to key audiences, enabling the Foundation to better serve its constituency in its mission to put an end to breast cancer. Help us spread the word about the Share Your Insight campaign by mailing the postcard we’ve included in this issue of Frontline to a friend. “As a patient advocacy organization, what better way for the Komen Foundation to understand the needs of breast cancer patients and their families and represent their views on breast cancer issues than to ask them directly?” said Susan Braun, president and chief executive officer of the Komen Foundation. “Share Your Insight is a real-time forum that allows patients, their families and anyone involved in the fight against breast cancer to tell us what they think about issues relevant to them and to our mission.” Visit www.komen.org to take the poll and have the option to join the Komen Foundation’s polling panel. -

The Cystinosis Advocate
Volume 5, Issue 2 P a g e 1 The Cystinosis Advocate Volume 5, Issue 2 Fall/Winter, 2012 Inside this issue: Sigma-Tau Pharmaceuticals, Inc. Receives President’s Message 2-3 FDA Approval of CYSTARAN™ Cystaran FAQ 4-5 GAITHERSBURG, MD, October 4, 2012—Sigma-Tau Pharmaceuticals, Inc. announced today that the Company has received approval from the U.S. Food & Drug Mexico Symposium 6-7 Administration (“FDA”) for CYSTARAN™ (cysteamine ophthalmic solution) 0.44%, a topical Financial Update 8-9 ophthalmic therapeutic, developed in partnership with the National Institutes of Health Family Support Update 10 (“NIH”), for the treatment of patients suffering from corneal cysteine crystal accumulation as a result of cystinosis. CYSTARAN™ is designated an Orphan Drug in the U.S. and has Family Story 10 been granted seven years of market exclusivity. Midwest Gathering 11 Cysteamine is a cysteine-depleting agent which lowers the cysteine content of cells in Research Update 12 patients with cystinosis. However, when New Studies Enrolling 13 orally administered, cysteamine does Celebration of Science 14-15 not reach the cornea and is therefore ineffective in reducing the ocular effects Rare Disease Conf. 16 of cystinosis. CYSTARAN™ is for topical Awareness Bracelets 17 ophthalmic use and is indicated for the RaptorCares 18-19 treatment of corneal cystine crystal accumulation in patients with cystinosis. Education Update 20 As a result, CYSTARAN™ now represents the only FDA-approved ophthalmic treatment for NORD Update 21 this condition. Development Update 22-23 “As a Company dedicated to the development and commercialization of novel therapies Utah Golf Fundraiser 24-25 that address the unmet medical needs of a wide range of rare disease patients, we are Family Story 26-27 delighted to announce the approval of CYSTARAN™,” noted Dave Lemus, Chief Operating (continued on page 4) Halloween Fundraiser 28-29 CRN Family Conference: Heroes Among Us July 18-20, 2013 in Washington, D.C. -

Fiscal Brief
New York City Independent Budget Office Fiscal Brief July 2021 A Full Accounting: How Much Does New York City Spend On Its Criminal Justice System? Summary Over the past year, much attention has focused on how much the city spends on policing. But the police department is only one part of a larger system responsible for law enforcement, including adjudication, and, when deemed appropriate, detention and other related functions. IBO has looked at the full cost of what is commonly known as the criminal justice system—from arrest to prosecution to incarceration and its alternatives—and tracked the changes in spending from 2001 through 2020. The report looks at the system as a whole as well as its component parts. Among our findings: • Spending by the agencies involved in the criminal justice system has grown from $5.1 billion in 2001 to $9.2 billion in 2020, an increase of 83 percent over two decades. When adjusted for inflation, though, the growth in spending is a far more modest 1.3 percent. • Funding from the city typically covers about 90 percent of the cost to support the system—$4.6 billion in city-generated funds in 2001 and $8.2 billion in 2020. • The police and correction departments have consistently absorbed most of the funds for the justice system. But over the past two decades, the two departments’ share of system spending has declined from 84 percent in 2001 to 79 percent in 2020. Conversely, despite the decline in the number of arrests over that period, there has been no corresponding decline in the share of criminal justice spending on the offices of the District Attorneys or Special Narcotics Prosecutor. -

PRESCRIPTION DRUG COUPON STUDY Report to the Massachusetts Legislature JULY 2020
COMMONWEALTH OF MASSACHUSETTS HEALTH POLICY COMMISSION PRESCRIPTION DRUG COUPON STUDY Report to the Massachusetts Legislature JULY 2020 EXECUTIVE SUMMARY In this report, required by Chapter 363 of the 2018 Session Prescription drug coupons are currently allowed in all 50 Laws, the Massachusetts Health Policy Commission (HPC) states for commercially-insured patients. Federal health examines the use and impact of prescription drug coupons insurance programs, such as Medicare, Medicaid, Tricare in Massachusetts. This report focuses on coupons issued by and Veteran’s Administration, prohibit the use of coupons pharmaceutical manufacturers that reduce a commercial based on federal anti-kickback statutes. Massachusetts patient’s cost-sharing. Prescription drug coupons are offered became the last state to authorize commercial coupon use almost exclusively on branded drugs, which comprise only in 2012 but continues to prohibit manufacturers from 10% of all prescriptions dispensed in the U.S., but account offering coupons and discounts on any prescription drug for 79% of total drug spending. Despite the immediate ben- that has an “AB rated” generic equivalent as determined efit of drug coupons to patients, policymakers and experts by the Food and Drug Administration (FDA). The 2012 debate whether and how coupons should be allowed in the law authorizing coupons in Massachusetts also contained commercial market given the potential relationship between a sunset provision, under which the law would have been coupon usage and increased spending on branded drugs repealed on July 1, 2015. However, this date of repeal versus lower cost alternatives. was postponed several times and ultimately extended to January 1, 2021. Massachusetts has long sought to con- Coupons reduce or eliminate the patient’s cost-sharing sider the impact of drug coupons on the Commonwealth’s responsibility required by the patient’s insurance plan, while landmark cost containment goals, as well as the benefits for the plan’s costs for the drug remain unchanged. -

Understanding Women's Self-Promotion
UNDERSTANDING WOMEN’S SELF-PROMOTION DETRIMENTS: THE BACKLASH AVOIDANCE MODEL by CORINNE ALISON MOSS-RACUSIN A dissertation submitted to the Graduate School-New Brunswick Rutgers, The State University of New Jersey In partial fulfillment of the requirements For the degree of Doctor of Philosophy Graduate Program in Psychology Written under the direction of Dr. Laurie A. Rudman And approved by ____________________ ____________________ ____________________ ____________________ New Brunswick, New Jersey May, 2011 ABSTRACT OF THE DISSERTATION Understanding Women’s Self-Promotion Detriments: The Backlash Avoidance Model. By CORINNE ALISON MOSS-RACUSIN Dissertation Director: Dr. Laurie A. Rudman Although self-promotion is necessary for career success, women experience backlash (i.e., social and economic penalties) for this behavior because it violates female gender stereotypes (Rudman, 1998). Moreover, women who fear backlash have difficulty with self-promotion, relative to men (Moss-Racusin & Rudman, 2010). The goal of this dissertation was to test the author’s backlash avoidance model (BAM), with the expectation that women’s beliefs that self-promotion violates female gender stereotypes lead them to fear backlash for this behavior, which in turn undermines their self- promotion abilities. Moreover, it was expected that the relationship between fear of backlash and self-promotion success would be at least partially mediated by self- regulatory focus (Crowe & Higgins, 1997) and perceived entitlement (Babcock & Laschever, 2003). To examine these ideas, Study 1 (N = 300) compared male and female participants’ performance on an essay-writing self-promotion task. As expected, women reported higher levels of fear of backlash and lower levels of self-promotion success than ii men. -
![Archons (Commanders) [NOTICE: They Are NOT Anlien Parasites], and Then, in a Mirror Image of the Great Emanations of the Pleroma, Hundreds of Lesser Angels](https://docslib.b-cdn.net/cover/8862/archons-commanders-notice-they-are-not-anlien-parasites-and-then-in-a-mirror-image-of-the-great-emanations-of-the-pleroma-hundreds-of-lesser-angels-438862.webp)
Archons (Commanders) [NOTICE: They Are NOT Anlien Parasites], and Then, in a Mirror Image of the Great Emanations of the Pleroma, Hundreds of Lesser Angels
A R C H O N S HIDDEN RULERS THROUGH THE AGES A R C H O N S HIDDEN RULERS THROUGH THE AGES WATCH THIS IMPORTANT VIDEO UFOs, Aliens, and the Question of Contact MUST-SEE THE OCCULT REASON FOR PSYCHOPATHY Organic Portals: Aliens and Psychopaths KNOWLEDGE THROUGH GNOSIS Boris Mouravieff - GNOSIS IN THE BEGINNING ...1 The Gnostic core belief was a strong dualism: that the world of matter was deadening and inferior to a remote nonphysical home, to which an interior divine spark in most humans aspired to return after death. This led them to an absorption with the Jewish creation myths in Genesis, which they obsessively reinterpreted to formulate allegorical explanations of how humans ended up trapped in the world of matter. The basic Gnostic story, which varied in details from teacher to teacher, was this: In the beginning there was an unknowable, immaterial, and invisible God, sometimes called the Father of All and sometimes by other names. “He” was neither male nor female, and was composed of an implicitly finite amount of a living nonphysical substance. Surrounding this God was a great empty region called the Pleroma (the fullness). Beyond the Pleroma lay empty space. The God acted to fill the Pleroma through a series of emanations, a squeezing off of small portions of his/its nonphysical energetic divine material. In most accounts there are thirty emanations in fifteen complementary pairs, each getting slightly less of the divine material and therefore being slightly weaker. The emanations are called Aeons (eternities) and are mostly named personifications in Greek of abstract ideas. -
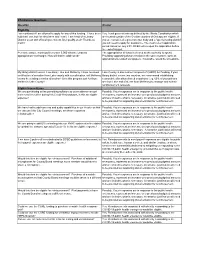
8/12/2020 Webinar Questions & Answers
8/12 Webinar Questions Question Answer Eligibility I am confused if I am allowed to apply for any of this funding. I have been Yes. Local governments as defined by the Illinois Constitution which told that I can, but I've also been told I can't. I am head of a Library are located outside of the 5-collar counties of Chicago are eligible. If District, so an unit of local government. Do I qualify at all? Thanks so you are considered a governmental body and a "special taxing district" much! you will need to apply for assistance. The most recent application period closed on July 24th. DCEO will re-open the application before the end of August. Per state statute, municipalities under 5,000 allocate funds by The appropriation of funds is viewed as the authority to spend. appropriation--not budget. How will that be addressed? Providing supporting documentation in the same manner, such as appropriations-related workpapers, if available, would be acceptable. My library district covers 2 counties; Lake and McHenry. I have received Lake County is also a direct recipient of CARES Act funding. If your certification information from Lake county with our allocation, will McHenry library district covers two counties, we recommend establishing county be sending a similar allocation? Or is this program just for those reasonable allocations based on patrons (e.g. 60% of your patrons entities in Lake County? are from Lake and 40% are from McHenry) to manage and submit reimbursement requests. Eligible Expenditures We are purchasing online permitting software so our residents can get Possibly. -

Cause-Related Marketing: a Critical Look at Marketplace Meeting
Running head: CAUSE-RELATED MARKETING 1 Cause-related Marketing A Critical Look at Marketplace Meeting Philanthropy Lindsey Winneroski A Senior Thesis submitted in partial fulfillment of the requirements for graduation in the Honors Program Liberty University Spring 2015 CAUSE-RELATED MARKETING 2 Acceptance of Senior Honors Thesis This Senior Honors Thesis is accepted in partial fulfillment of the requirements for graduation from the Honors Program of Liberty University. ______________________________ Lynnda S. Beavers, Ph.D. Thesis Chair ______________________________ George Young, Ph.D. Committee Member ______________________________ Amy Bonebright, M.A. Committee Member ______________________________ Brenda Ayres, Ph.D. Honors Director ______________________________ Date CAUSE-RELATED MARKETING 3 Abstract The following discussion offers a critical look at cause-related marketing (CRM), a strategic partnership between a corporate and nonprofit entity in which a portion of product sales, or a one-time donation, is given in support of a cause. CRM is an extension of a corporation’s social responsibility efforts in a push to meet increasing consumer demand for organizational accountability and social-consciousness. The discussion examines factors that have fed the mandate for corporate social responsibility, including a connection through online platforms and a generational cohort with a demand to “give back.” Research shows benefits of implementing CRM; however, many ethical issues must be considered when organizations attempt to blend for-profit motives with altruism. CRM and its impact on the definition of philanthropy will be evaluated through the investigation of two campaigns—the Susan G. Komen Pink Campaign and the ALS ice bucket challenge. CAUSE-RELATED MARKETING 4 Cause-related Marketing A Critical Look at Marketplace Meeting Philanthropy In today’s marketplace, consumers expect businesses not only to meet individuals’ needs through products or services but also to be socially responsible by giving back to society. -
Fringe Season 1 Transcripts
PROLOGUE Flight 627 - A Contagious Event (Glatterflug Airlines Flight 627 is enroute from Hamburg, Germany to Boston, Massachusetts) ANNOUNCEMENT: ... ist eingeschaltet. Befestigen sie bitte ihre Sicherheitsgürtel. ANNOUNCEMENT: The Captain has turned on the fasten seat-belts sign. Please make sure your seatbelts are securely fastened. GERMAN WOMAN: Ich möchte sehen wie der Film weitergeht. (I would like to see the film continue) MAN FROM DENVER: I don't speak German. I'm from Denver. GERMAN WOMAN: Dies ist mein erster Flug. (this is my first flight) MAN FROM DENVER: I'm from Denver. ANNOUNCEMENT: Wir durchfliegen jetzt starke Turbulenzen. Nehmen sie bitte ihre Plätze ein. (we are flying through strong turbulence. please return to your seats) INDIAN MAN: Hey, friend. It's just an electrical storm. MORGAN STEIG: I understand. INDIAN MAN: Here. Gum? MORGAN STEIG: No, thank you. FLIGHT ATTENDANT: Mein Herr, sie müssen sich hinsetzen! (sir, you must sit down) Beruhigen sie sich! (calm down!) Beruhigen sie sich! (calm down!) Entschuldigen sie bitte! Gehen sie zu ihrem Sitz zurück! [please, go back to your seat!] FLIGHT ATTENDANT: (on phone) Kapitän! Wir haben eine Notsituation! (Captain, we have a difficult situation!) PILOT: ... gibt eine Not-... (... if necessary...) Sprechen sie mit mir! (talk to me) Was zum Teufel passiert! (what the hell is going on?) Beruhigen ... (...calm down...) Warum antworten sie mir nicht! (why don't you answer me?) Reden sie mit mir! (talk to me) ACT I Turnpike Motel - A Romantic Interlude OLIVIA: Oh my god! JOHN: What? OLIVIA: This bed is loud. JOHN: You think? OLIVIA: We can't keep doing this. -

A Cultural Analysis of a Physicist ''Trio'' Supporting the Backlash Against
ARTICLE IN PRESS Global Environmental Change 18 (2008) 204–219 www.elsevier.com/locate/gloenvcha Experiences of modernity in the greenhouse: A cultural analysis of a physicist ‘‘trio’’ supporting the backlash against global warming Myanna Lahsenà Center for Science and Technology Policy Research, University of Colorado and Instituto Nacional de Pesquisas Epaciais (INPE), Av. dos Astronautas, 1758, Sa˜o Jose´ dos Campos, SP 12227-010 Brazil Received 18 March 2007; received in revised form 5 October 2007; accepted 29 October 2007 Abstract This paper identifies cultural and historical dimensions that structure US climate science politics. It explores why a key subset of scientists—the physicist founders and leaders of the influential George C. Marshall Institute—chose to lend their scientific authority to this movement which continues to powerfully shape US climate policy. The paper suggests that these physicists joined the environmental backlash to stem changing tides in science and society, and to defend their preferred understandings of science, modernity, and of themselves as a physicist elite—understandings challenged by on-going transformations encapsulated by the widespread concern about human-induced climate change. r 2007 Elsevier Ltd. All rights reserved. Keywords: Anti-environmental movement; Human dimensions research; Climate change; Controversy; United States; George C. Marshall Institute 1. Introduction change itself, what he termed a ‘‘strong theory of culture.’’ Arguing that the essential role of science in our present age Human Dimensions Research in the area of global only can be fully understood through examination of environmental change tends to integrate a limited con- individuals’ relationships with each other and with ‘‘mean- ceptualization of culture. -

Mechanical and Stress-Optical Properties of Photoelastic Materials
MECHANICAL AND STRESS-OPTICAL PROPERTIES OF PHOTOELAS!IC MATERIALS by YOSHIO TESHIMA A THESIS submitted to OREGON STATE COLLEGE in partial fulfillment of the requirements for the degree or MASTER OF SCIENCE June 1953 l}tSffiDr Redacted for Privacy rrfutrut S;ofr*rur l'f lrrlrut*l h6lnrslrs E ODugr cf lrJou Redacted for Privacy Redacted for Privacy @le.rr Of Eohoot 6rrdu*r Redacted for Privacy Erta Sr;1r lr Dror.aid !tp.C E {rllr f. .hm ,, : ACKNOWLEOOMENT Sincere appreciation is expressed to H. D. Christensen, Assistant Professor of Mechani cal Engineering, who sug eated the thesis subject, and under. whose direction this thesis was com pleted. TABLE OF CONTENTS Page I. I NTRODUCTION • • • • • • • • • • • • • • • • • l II. THE PROTOBLASTIC METHOD OF STRESS ANALYSIS • • 4 III. BASIC REQUIREMENTS OF PHOTOELASTIC MATERIALS • 9 IV. PHOTOELASTIC MATERIAI,S • • • • • • • • • • • • 1. Gl ass • • • • • • • • • • • • • • • • • • • 15 2. Cellu1oid • • • • • • • • • •. • • • • • • • 16 3. Cata1in 61-893 • • • • • • • • • • • • • • 18 4. Fosterite • • • • • • • • • • • • • • • • • 21 5. Homa1ite CR-39 _ • • • • • • • • • • • • • • 22 6. Y...ris ton • • • • • • • • • • • • • • • • • • 24 7. Pl ex1g1nss and Lucite • • • • • • • • • • • 25 6. l!arb1ette • • • • • • • • • • • • • • • • • 26 9. Viny11te • • • • • • • • • • • • • • • • • 27 10. Summary of the Properties of Photoelastie Materials • • • • • • • • • • • • • • • • ~ 26 V. CASTING ND TESTING SEL: ~CTED POLYESTER RESINS • 31 1 • . Selection of Resins • • • • • • • • • • •. • 34 2. Mixing the