(Annex3) (Last Updated: 8 January 2021) Note: Underlined Regions
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Cadmium Contamination in Orchard Soils and Fruit Trees and Its Potential Health Risk in Guangzhou, China
ARTICLE IN PRESS + MODEL http://www.paper.edu.cn Environmental Pollution xx (2005) 1e7 Cadmium contamination in orchard soils and fruit trees and its potential health risk in Guangzhou, China J.T. Li a, J.W. Qiu b, X.W. Wang a, Y. Zhong a, C.Y. Lan a,*, W.S. Shu a,* a School of Life Sciences and State Key Laboratory of Biocontrol, Sun Yat-sen (Zhongshan) University, Guangzhou 510275, PR China b Department of Biology, Hong Kong Baptist University, Kowloon, Hong Kong, PR China Received 6 August 2005; received in revised form 19 September 2005; accepted 14 October 2005 Carambola fruit can accumulate high levels of cadmium and may be a health risk for humans. Abstract This study examines cadmium (Cd) contamination in orchard soils and fruit trees in Guangzhou, China, and assesses its potential health risk. Soils and tissues samples of three species of fruit trees were collected from three orchards. The average soil Cd concentration was 1.27, 1.84 and 0.68 mg/kg in orchards I, II, and III, respectively. The carambola (Averrhoa carambola) accumulated exceptionally high concentrations of Cd (7.57, 10.84, 9.01 and 2.15 mg/kg dw in root, twig, leaf and fruit, respectively), being 6.0e24 times and 4.0e10 times the corresponding tissue Cd in the longan (Dimocarpus longan) and wampee (Clausena lansium), respectively. Furthermore, all Cd concentrations (0.04e0.25 mg Cd/kg fw) of the fruits exceeded the tolerance limit of cadmium in foods of PR China (0.03 mg/kg fw). Our results indicate that the carambola tree has high Cd accumulation capacity and might be a Cd accumulator; and its fruit, among the three species of fruits studied, also poses the highest potential health risk to local residents. -

A Refugium for Scaevola Plumieri, a Highly Threatened Rare Plant in Sri Lanka
Cey. J. Sci. (Bio. Sci.) Vol 34, 2005, 21-25 21 A REFUGIUM FOR SCAEVOLA PLUMIERI, A HIGHLY THREATENED RARE PLANT IN SRI LANKA A.H.Magdon Jayasuriya Environment & Management Lanka, 68 Davidson Road, Colombo 4, Sri Lanka ABSTRACT Scaevola plumieri ( “Hin-takkada”), a surface-creeping shrub among strand vegetation, is extremely rare and “highly threatened” having been previously recorded from no more than six locations in Sri Lanka, mostly during the 19th century. The species is presumed to be extinct in its 19th century localities on the west and east coasts due to loss of habitat, and in its 20th century localities on the east coast due to the recent Tsunami disaster. The Puttalam lagoon shore within the Wilpattu National Park, where this plant was recently located, will therefore be a refugium for this species. Incidentally, this is also the first time that this plant was observed on a lagoon-shore habitat in Sri Lanka, instead of its usual sea shore habitat. This indicates the high conservation value of the Wilpattu beach front of the Kala Oya Lower Basin which also harbours one of the last remaining pristine stretches of mangroves in Sri Lanka, which should be noted by the Department of Wildlife Conservation in park planning and management. INTRODUCTION Scaevola plumieri (L.) Vahl belongs to the activities in coastal zones, “ Hin-takkada” populations have presumably disappeared from the family Goodeniaceae and its local name is th (“Hin-takkada”). It is a surface-creeping above localities. The specimens recorded in the 20 (prostate) shrub with rounded and leathery century were gathered between 1969 – 1974 only (coriaceaous) leaves not exceeding 8 cm in from two localities on the lower east coast, namely length, while the fruit (drupe) is red when ripe. -

Mapping Resistance to Stem Rust, Stripe Rust, and Powdery Mildew, and Genotype Screening of Breeding Germplasm for Disease Resistance
ABSTRACT VANGESSEL, CARL JOSEPH. Disease Resistance in Winter Wheat: Mapping Resistance to Stem Rust, Stripe Rust, and Powdery Mildew, and Genotype Screening of Breeding Germplasm for Disease Resistance. (Under the direction of Dr. David Marshall). Wheat production worldwide faces many challenges including fungal pathogens which can reduce growth, nutrient content, and yield. As the global population continues to increase, it is important to maximize production of wheat which provides a significant proportion of the human diet. Wheat breeders and pathologists are addressing the pathogen threat to wheat by breeding cultivars with improved resistance. However, as pathogen populations mutate and overcome resistance, it is important to identify sources of resistance to new pathogen races and understand the genetics of resistance in commercial cultivars. Stem rust (Puccinia graminis), stripe rust (Puccinia striiformis), and powdery mildew (Blumeria graminis) are among the most common and aggressive pathogens of wheat occurring worldwide. The stem rust resistance gene Sr31 was reliably effective until being overcome in 1999 by the race Ug99. This new race of stem rust has since evolved to include 12 related races and are potentially virulent to a majority of commercially produced wheat globally. The soft red winter wheat (SRWW) line, MD01W28- 08-11, was identified as having adult plant resistance (APR) to Ug99 in Njoro, Kenya. This line was crossed with the susceptible SRWW cultivar Coker 9553 and the subsequent 279 doubled haploid (DH) population used for linkage mapping analysis to better characterize the source of stem rust APR. A linkage map with 3,159 SNPs was produced that identified a significant quantitative trait loci (QTL) on the short arm of chromosome 6D and two QTL on the long arms of chromosomes 2B and 4B. -

Known Host Plants of Huanglongbing (HLB) and Asian Citrus Psyllid
Known Host Plants of Huanglongbing (HLB) and Asian Citrus Psyllid Diaphorina Liberibacter citri Plant Name asiaticus Citrus Huanglongbing Psyllid Aegle marmelos (L.) Corr. Serr.: bael, Bengal quince, golden apple, bela, milva X Aeglopsis chevalieri Swingle: Chevalier’s aeglopsis X X Afraegle gabonensis (Swingle) Engl.: Gabon powder-flask X Afraegle paniculata (Schum.) Engl.: Nigerian powder- flask X Atalantia missionis (Wall. ex Wight) Oliv.: see Pamburus missionis X X Atalantia monophylla (L.) Corr.: Indian atalantia X Balsamocitrus dawei Stapf: Uganda powder- flask X X Burkillanthus malaccensis (Ridl.) Swingle: Malay ghost-lime X Calodendrum capense Thunb.: Cape chestnut X × Citroncirus webberi J. Ingram & H. E. Moore: citrange X Citropsis gilletiana Swingle & M. Kellerman: Gillet’s cherry-orange X Citropsis schweinfurthii (Engl.) Swingle & Kellerm.: African cherry- orange X Citrus amblycarpa (Hassk.) Ochse: djerook leemo, djeruk-limau X Citrus aurantiifolia (Christm.) Swingle: lime, Key lime, Persian lime, lima, limón agrio, limón ceutí, lima mejicana, limero X X Citrus aurantium L.: sour orange, Seville orange, bigarde, marmalade orange, naranja agria, naranja amarga X Citrus depressa Hayata: shiikuwasha, shekwasha, sequasse X Citrus grandis (L.) Osbeck: see Citrus maxima X Citrus hassaku hort. ex Tanaka: hassaku orange X Citrus hystrix DC.: Mauritius papeda, Kaffir lime X X Citrus ichangensis Swingle: Ichang papeda X Citrus jambhiri Lushington: rough lemon, jambhiri-orange, limón rugoso, rugoso X X Citrus junos Sieb. ex Tanaka: xiang -

List of the Import Prohibited Plants
List of the Import Prohibited Plants The Annexed Table 2 of the amended Enforcement Ordinance of the Plant Protection Law (Amended portions are under lined) Districts Prohibited Plants Quarantine Pests 1. Yemen, Israel, Saudi Arabia, Fresh fruits of akee, avocado, star berry, Mediterranean fruit fly Syria, Turkey, Jordan, Lebanon, allspice, olive, cashew nut, kiwi fruit, Thevetia (Ceratitis capitata) Albania, Italy, United Kingdom peruviana, carambola, pomegranate, jaboticaba, (Great Britain and Northern broad bean, alexandrian laurel, date palm, Ireland, hereinafter referred to as Muntingia calabura, feijoa, pawpaw, mammee "United Kingdom"), Austria, apple, longan, litchi, and plants of the genera Netherlands, Cyprus, Greece, Ficus, Phaseolus, Diospyros(excluding those Croatia, Kosovo, Switzerland, listed in appendix 41), Carissa, Juglans, Morus, Spain, Slovenia, Serbia, Germany, Coccoloba, Coffea, Ribes, Vaccinium, Hungary, France, Belgium, Passiflora, Dovyalis, Ziziphus, Spondias, Musa Bosnia and Herzegovina, (excluding immature banana), Carica (excluding Portugal, Former Yugoslav those listed in appendix 1), Psidium, Artocarpus, Republic of Macedonia, Malta, , Annona, Malpighia, Santalum, Garcinia, Vitis Montenegro, Africa, Bermuda, (excluding those listed in appendices 3 and 54), Argentina, Uruguay, Ecuador, El Eugenia, Mangifera (excluding those listed in Salvador, Guatemala, Costa Rica, appendices 2 ,36 ,43 ,51 and 53), Ilex, Colombia, Nicaragua, West Indies Terminalia and Gossypium, and Plants of the (excluding Cuba, Dominican family Sapotaceae, Cucurbitaceae (excluding Republic,Puerto Rico), Panama, those listed in appendices 3 and 42), Cactaceae Paraguay, Brazil, Venezuela, (excluding those listed in appendix 35), Peru, Bolivia, Honduras, Australia Solanaceae (excluding those listed in (excluding Tasmania), Hawaiian appendices 3 and 42), Rosaceae (excluding Islands those listed in appendices 3 and 31) and Rutaceae (excluding those listed in appendices 4 to 8 ,39 ,45 and 56). -
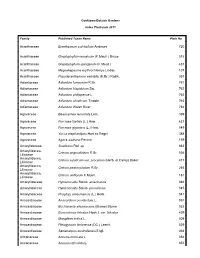
Abelmoschus Moschatus Subsp
Cooktown Botanic Gardens Index Plantarum 2011 Family Published Taxon Name Plate No Acanthaceae Eranthemum pulchellum Andrews 720 Acanthaceae Graptophyllum excelsum (F.Meull.) Druce 515 Acanthaceae Graptophyllum spinigerum (F.Meull.) 437 Acanthaceae Megaskepasma erythrochlamys Lindau 107 Acanthaceae Pseuderanthemum variabile (R.Br.) Radlk. 357 Adiantaceae Adiantum formosum R.Br. 761 Adiantaceae Adiantum hispidulum Sw. 762 Adiantaceae Adiantum philippense L. 765 Adiantaceae Adiantum silvaticum Tindale 763 Adiantaceae Adiantum Walsh River 764 Agavaceae Beaucarnea recurvata Lem. 399 Agavaceae Furcraea foetida (L.) Haw. 637 Agavaceae Furcraea gigantea (L.) Haw. 049 Agavaceae Yucca elephantipes Hort.ex Regel 388 Agavaceae Agave sisalana Perrine. 159 Amarylidaceae Scadoxus Raf. sp 663 Amaryllidacea, Crinum angustifolium R.Br. 536 Liliaceae Amaryllidacea, Crinum asiaticum var. procerum (Herb. et Carey) Baker 417 Liliaceae Amaryllidacea, Crinum pedunculatum R.Br. 265 Liliaceae Amaryllidacea, Crinum uniflorum F.Muell. 161 Liliaceae Amaryllidaceae Hymenocallis Salisb. americanus 046 Amaryllidaceae Hymenocallis Salisb. peruvianna 045 Amaryllidaceae Proiphys amboinensis (L.) Herb. 041 Anacardiaceae Anacardium occidentale L. 051 Anacardiaceae Buchanania arborescens (Blume) Blume. 022 Anacardiaceae Euroschinus falcatus Hook.f. var. falcatus 429 Anacardiaceae Mangifera indica L. 009 Anacardiaceae Pleiogynium timorense (DC.) Leenh. 029 Anacardiaceae Semecarpus australiensis Engl. 368 Annonaceae Annona muricata L. 054 Annonaceae Annona reticulata L. 053 Annonaceae Annona squamosa 602 Annonaceae Cananga odorata (Lam.) Hook.f.&Thomson 406 Annonaceae Melodorum leichhardtii (F.Muell.) Diels. 360 Annonaceae Rollinia deliciosa Saff. 098 Apiaceae Centella asiatica (L.) Urb. 570 Apocynaceae Adenium obesum (Forssk.) Roem. & Schult. 489 Apocynaceae Allamanda cathartica L. 047 Apocynaceae Allamanda violacea Gardn. & Field. 048 Apocynaceae Alstonia actinophylla (A.Cunn.) K.Schum. 026 Apocynaceae Alstonia scholaris (L.) R.Br. 012 Apocynaceae Alyxia ruscifolia R.Br. -

Distribution and Frequency of Wheat Stem Rust Races (Puccinia Graminis F
©2018 Scienceweb Publishing Journal of Agricultural and Crop Research Vol. 6(5), pp. 88-96, November 2018 ISSN: 2384-731X Research Paper Distribution and frequency of wheat stem rust races (Puccinia graminis f. sp. tritici) in Ethiopia Netsanet Bacha Hei1* • Tsegaab Tesfaye1 • Getaneh Woldeab1 • Endale Hailu1 • Bekele Hundie2 • Daniel Kassa2 • Fikirte Yirga2 • Fufa Anbessa3 • Wubishet Alemu4 • Teklay Abebe5 • Miruts Legesse5 • Alemar Seid6 • Tesfaye Gebrekirstos7 1Ethiopian Institute of Agricultural Research, Ambo Plant Protection Research Center, Ambo, Ethiopia. 2Ethiopian Institute of Agricultural Research, Kulumsa Agricultural Research Center, Assela, Ethiopia. 3Oromia Agricultural Research Institute, Bore Agricultural Research Center, Bore, Ethiopia. 4Oromia Agricultural Research Institute, Sinana Agricultural Research Center, Bale Robe, Ethiopia. 5Tigray Agricultural Research Institute, Alamata Agricultural Research Center, Alamata, Ethiopia. 6Southern Agricultural Research Institute, Areka Agricultural Research Center, Ethiopia. 7Tigray Agricultural Research Institute, Mekele Agricultural Research Center, Mekele, Ethiopia. *Corresponding author. E-mail: [email protected]. Accepted 6th July, 2018 Abstract. Stem rust caused by Puccinia graminis f. sp. tritici (Pgt) is one of the most important diseases of wheat in Ethiopia. The stem rust pathogen is capable to rapidly develop new virulence to resistance genes owing to mutation and genetic recombination. Ethiopian highlands are known hot spots for the rapid evolution and spread of new rust races. The present study was conducted to investigate the virulence diversity and spatial distribution of races of Pgt in the major wheat growing areas of Ethiopia. The physiologic races of the rust fungus were determined on seedlings of the standard wheat stem rust differentials following the international system of nomenclature. Three hundred and forty- seven samples were analyzed in 2014 and 2015 cropping seasons from Oromia, Amhara, Tigray and Southern Nations and Nationalities Peoples’ regions. -

Scaevola Taccada (Gaertn.) Roxb
BioInvasions Records (2021) Volume 10, Issue 2: 425–435 CORRECTED PROOF Rapid Communication First record of naturalization of Scaevola taccada (Gaertn.) Roxb. (Goodeniaceae) in southeastern Mexico Gonzalo Castillo-Campos1,*, José G. García-Franco2 and M. Luisa Martínez2 1Red de Biodiversidad y Sistemática, Instituto de Ecología, A.C., Xalapa, Veracruz, 91073, México 2Red de Ecología Funcional, Instituto de Ecología, A.C. Xalapa, Veracruz, 91073, México Author e-mails: [email protected] (GCC), [email protected] (JGGF), [email protected] (MLM) *Corresponding author Citation: Castillo-Campos G, García- Franco JG, Martínez ML (2021) First Abstract record of naturalization of Scaevola taccada (Gaertn.) Roxb. (Goodeniaceae) Scaevola taccada (Gaertn.) Roxb. is native of Asia and eastern Africa but has been in southeastern Mexico. BioInvasions introduced into the Americas as an ornamental urban plant. This paper reports, for Records 10(2): 425–435, https://doi.org/10. the first time, the presence of Scaevola taccada in natural environments from 3391/bir.2021.10.2.21 southeastern Mexico. Several populations of S. taccada were identified during a Received: 23 July 2020 botanical survey of the coastal dunes of the Cozumel Island Biosphere Reserve Accepted: 22 October 2020 (State of Quintana Roo, Mexico) aimed at recording the most common plant Published: 22 January 2021 species. Scaevola taccada is considered as an invasive species of coastal areas in this region. Evidence of its invasiveness is suggested by the fact that populations Handling editor: Oliver Pescott consisting of individuals of different size classes are found distributed throughout Thematic editor: Stelios Katsanevakis the island. Furthermore, they appear to belong to different generations since we Copyright: © Castillo-Campos et al. -
A Voyage to China and the East Indies
PS708 .08 1771 v.1 VOYAGEA T O CHINA AND THE EAST INDIES, By PETER OSBECK, Rector of Hasloef and Woxtorp, Member of the Academy of Stockholm, and of the Society of U?jal. Together with A VOYAGE TO SURATTE, By OLOF TOREEN, Chaplain of the Gothic Lion East Indiaman. and An Accountof the CHINESE HUSBANDRY, By Captain CHARLES GUSTAVUS ECKEBERG. Tranflated from the German, By JOHN REINHOLD FORSTER, F.A.S. To which sre addci, A Faunula and Flora Sinensis, IN TWQ VOLUMES. VOL. I. LONDON, fdated for BENJAMIN WHITE, at Horace's Head, in Fleet-ftreet. M DCC LXXI. ' THOMAS PENNANT, E% O F DOWNING, in FLINTSHIRE, Dear Sir,- TH E peculiar obligations your good- nefs has laid me under, have left me no room to hefitate one moment in the choice of a patrOn for this publica- tion. This work was undertaken with your* approbation, enriched by you with many important additions, and has often been the fubjedt. of our converfation. But my obligations to you are not confined to the affiftance you have afford- ed in me this prefent work : by your fa- vour, I, who was an utter lb-anger to a 2 this : [iv] this country, have been introduced to a number of munificent and worthy friends, whofe acquaintance is both my honour and my happinefs. The fimilitude of our ftudies was what firft recommended me to your no- tice ; but your humanity was engaged to receive me to a nearer intimacy from a circumftance, which too frequently would have been the caufe of neglect the diftrefles I. -

Society for Growing Australian Plants, Cairns Branch
Society for Growing Australian Plants, Cairns Branch Newsletter 147 March 2015 In this issue… EXCURSION REPORT – STONEY CREEK, FEBRUARY 2015 ............................... 1 ANNUAL GENERAL MEETING NOTIFICATION .. 4 EXCURSION REPORT (CONTINUED FROM PAGE 3).................................... 5 SPECIES LIST : STONEY CREEK BELOW FALLS AND RAILWAY ...................... 5 WHAT’S HAPPENING… ...... 9 CAIRNS SGAP ................ 9 TABLELANDS SGAP .......... 9 EXCURSION REPORT – STONEY CREEK , FEBRUARY 2015 TOWNSVILLE SGAP ......... 9 Boyd Lenne I arrived at Stoney Creek hopeful that one of our resident experts would be in attendance. Needless to say I was overjoyed to find Bob Jago unfolding his tea table for a pre walk cuppa. We were soon joined by Coralie Stuart and Anne Mohun. Anne and I had both come from a Treeforce planting event that morning, so we felt very intrepid to make the extra mission to a SGAP event all afternoon. In no time at all we four were huddled under a small interpretive shelter chatting, and playing "how many trees can Bob name while sitting down over smoko". The rain abated, and we were off. At the carpark I was interested in a striking prodigious orange fig with an attractive leaf and undulate leaf margin. Bob informed me this was the Ficus virgata, var. virgata . Trip trapping over the bridge, we saw the incredibly dainty Maesa haplobotrys , displaying fruit and flowers at all stages. Very attractive. The waters below were speckled with bright Syzygium luehmannii fruit. Up the hill, and water was playing across the path. Bob demonstrated the Rhodamnia spongiosa , with its incredibly pronounced three veined leaf. Myrtaceae are not particularly known for their three veined leaf structure, and I mused about whether many of the FNQ Lauraceae displayed this structural diagnostic. -

Averrhoa Bilimbi 1 Averrhoa Bilimbi
Averrhoa bilimbi 1 Averrhoa bilimbi Averrhoa bilimbi Scientific classification Kingdom: Plantae (unranked): Angiosperms (unranked): Eudicots (unranked): Rosids Order: Oxalidales Family: Oxalidaceae Genus: Averrhoa Species: A. bilimbi Binomial name Averrhoa bilimbi L. Averrhoa bilimbi 2 Averrhoa bilimbi (commonly known as bilimbi, cucumber tree, or tree sorrel) is a fruit-bearing tree of the genus Averrhoa, family Oxalidaceae. It is a close relative of the carambola. Nomenclature The tree and fruit are known by different names in different languages.[1] They should not be confused with the carambola, which also share some of the same names despite being very different fruits. Balimbing in the Philippines actually refer to carambola and not bilimbi (which they call iba in Cebuano and kamias in Tagalog). Averrhoa bilimbi fruit Country Name English cucumber tree or tree sorrel India bilimbi,Irumban Puli,Chemmeen Puli,bimbul Sri Lanka Bilincha, bimbiri Dominican Republic Vinagrillo Philippines kamias,kalamias, or iba Malaysia belimbing asam, belimbing buloh, b'ling, or billing-billing Indonesia belimbing wuluh or belimbing sayur Thailand taling pling, or kaling pring Vietnam khế tàu Haiti blimblin Jamaica bimbling plum Cuba grosella china El Salvador & Nicaragua mimbro Costa Rica mimbro or tirigur Venezuela vinagrillo Surinam and Guyana birambi Argentina pepino de Indias France carambolier bilimbi or cornichon des Indes Seychelles bilenbi Averrhoa bilimbi 3 Distribution and habitat Possibly originating on the Moluccas, Indonesia, the species is cultivated or found semi-wild throughout Indonesia, The Philippines, Sri Lanka, Bangladesh, Myanmar (Burma) and Malaysia. It is common in other Southeast Asian countries. In India, where it is usually found in gardens, the bilimbi has gone wild in the warmest regions of the country. -

Healthy Food Traditions of Asia: Exploratory Case Studies From
Harmayani et al. Journal of Ethnic Foods (2019) 6:1 Journal of Ethnic Foods https://doi.org/10.1186/s42779-019-0002-x ORIGINALARTICLE Open Access Healthy food traditions of Asia: exploratory case studies from Indonesia, Thailand, Malaysia, and Nepal Eni Harmayani1, Anil Kumar Anal2, Santad Wichienchot3, Rajeev Bhat4, Murdijati Gardjito1, Umar Santoso1, Sunisa Siripongvutikorn5, Jindaporn Puripaatanavong6 and Unnikrishnan Payyappallimana7* Abstract Asia represents rich traditional dietary diversity. The rapid diet transition in the region is leading to a high prevalence of non-communicable diseases. The aim of this exploratory study was to document traditional foods and beverages and associated traditional knowledge that have potential positive health impacts, from selected countries in the region. The study also focused on identifying their importance in the prevention and management of lifestyle-related diseases and nutritional deficiencies as well as for the improvement of the overall health and wellbeing. This was conducted in selected locations in Indonesia, Thailand, Malaysia and Nepal through a qualitative method with a pre-tested documentation format. Through a detailed documentation of their health benefits, the study tries to highlight the significance of traditional foods in public health as well as their relevance to local market economies towards sustainable production and consumption and sustainable community livelihoods. Keywords: Traditional foods, Ethnic recipes, Asian health food traditions, Cultural dietary diversity, Indonesia, Thailand, Malaysia and Nepal Introduction Due to the dynamic adaptations to local biocultural con- Asia represents vast geographic, socioeconomic, bio- texts and refinement over generations through empirical logical, and cultural diversity. This is also reflected in the observations, they assume to have positive health impacts dietary diversity of traditional foods.