Probosciger Aterrimus), the Legacy of Landscape and Biogeographic History
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
Abelmoschus Moschatus Subsp
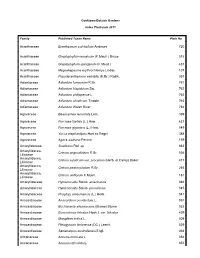
Cooktown Botanic Gardens Index Plantarum 2011 Family Published Taxon Name Plate No Acanthaceae Eranthemum pulchellum Andrews 720 Acanthaceae Graptophyllum excelsum (F.Meull.) Druce 515 Acanthaceae Graptophyllum spinigerum (F.Meull.) 437 Acanthaceae Megaskepasma erythrochlamys Lindau 107 Acanthaceae Pseuderanthemum variabile (R.Br.) Radlk. 357 Adiantaceae Adiantum formosum R.Br. 761 Adiantaceae Adiantum hispidulum Sw. 762 Adiantaceae Adiantum philippense L. 765 Adiantaceae Adiantum silvaticum Tindale 763 Adiantaceae Adiantum Walsh River 764 Agavaceae Beaucarnea recurvata Lem. 399 Agavaceae Furcraea foetida (L.) Haw. 637 Agavaceae Furcraea gigantea (L.) Haw. 049 Agavaceae Yucca elephantipes Hort.ex Regel 388 Agavaceae Agave sisalana Perrine. 159 Amarylidaceae Scadoxus Raf. sp 663 Amaryllidacea, Crinum angustifolium R.Br. 536 Liliaceae Amaryllidacea, Crinum asiaticum var. procerum (Herb. et Carey) Baker 417 Liliaceae Amaryllidacea, Crinum pedunculatum R.Br. 265 Liliaceae Amaryllidacea, Crinum uniflorum F.Muell. 161 Liliaceae Amaryllidaceae Hymenocallis Salisb. americanus 046 Amaryllidaceae Hymenocallis Salisb. peruvianna 045 Amaryllidaceae Proiphys amboinensis (L.) Herb. 041 Anacardiaceae Anacardium occidentale L. 051 Anacardiaceae Buchanania arborescens (Blume) Blume. 022 Anacardiaceae Euroschinus falcatus Hook.f. var. falcatus 429 Anacardiaceae Mangifera indica L. 009 Anacardiaceae Pleiogynium timorense (DC.) Leenh. 029 Anacardiaceae Semecarpus australiensis Engl. 368 Annonaceae Annona muricata L. 054 Annonaceae Annona reticulata L. 053 Annonaceae Annona squamosa 602 Annonaceae Cananga odorata (Lam.) Hook.f.&Thomson 406 Annonaceae Melodorum leichhardtii (F.Muell.) Diels. 360 Annonaceae Rollinia deliciosa Saff. 098 Apiaceae Centella asiatica (L.) Urb. 570 Apocynaceae Adenium obesum (Forssk.) Roem. & Schult. 489 Apocynaceae Allamanda cathartica L. 047 Apocynaceae Allamanda violacea Gardn. & Field. 048 Apocynaceae Alstonia actinophylla (A.Cunn.) K.Schum. 026 Apocynaceae Alstonia scholaris (L.) R.Br. 012 Apocynaceae Alyxia ruscifolia R.Br. -

Recommended Band Size List Page 1
Jun 00 Australian Bird and Bat Banding Scheme - Recommended Band Size List Page 1 Australian Bird and Bat Banding Scheme Recommended Band Size List - Birds of Australia and its Territories Number 24 - May 2000 This list contains all extant bird species which have been recorded for Australia and its Territories, including Antarctica, Norfolk Island, Christmas Island and Cocos and Keeling Islands, with their respective RAOU numbers and band sizes as recommended by the Australian Bird and Bat Banding Scheme. The list is in two parts: Part 1 is in taxonomic order, based on information in "The Taxonomy and Species of Birds of Australia and its Territories" (1994) by Leslie Christidis and Walter E. Boles, RAOU Monograph 2, RAOU, Melbourne, for non-passerines; and “The Directory of Australian Birds: Passerines” (1999) by R. Schodde and I.J. Mason, CSIRO Publishing, Collingwood, for passerines. Part 2 is in alphabetic order of common names. The lists include sub-species where these are listed on the Census of Australian Vertebrate Species (CAVS version 8.1, 1994). CHOOSING THE CORRECT BAND Selecting the appropriate band to use combines several factors, including the species to be banded, variability within the species, growth characteristics of the species, and band design. The following list recommends band sizes and metals based on reports from banders, compiled over the life of the ABBBS. For most species, the recommended sizes have been used on substantial numbers of birds. For some species, relatively few individuals have been banded and the size is listed with a question mark. In still other species, too few birds have been banded to justify a size recommendation and none is made. -

Dwi Astuti Zoological Division, R.C
Phylogenetic relationships of cockatoos (Aves: Psittaciformes) based on DNA sequences of the seventh intron of nuclear β-fibrinogen gene Dwi Astuti Zoological Division, R.C. for Biology - Indonesian Institute of Sciences, Cibinong Science Centre Indonesia [email protected] O Cockatoos are belonged to Cacatuinae (Forshaw, 1989) order : Psittaciformes, Cockatoo Distribution family : Psittacidae (Forshaw, 1989) Calopsittacini Chalyptorhynchini Cacatuini Cacatuidae (del Hoyo,1998) Nymphicus subfamily: Cacatuinae Probosciger Calyptorhynchus Challocephalon Eolophus Cacatua tribes : Calopsittacini Chalyptorhynchini Cacatuini E. roseicapillus Probosciger N. hollandicus P. aterrimus C. baudinii C. fimbriatum C. sulphurea O In the world, there are six extant genera C. latirostris C.galerita Eolophus C. lathami C. alba Cacatua consisting of 21 cockatoo species C. banksii Nymphicus C. moluccensis leadbeateri C. funereus C. goffini Calyptorhynchus O Some previous authors have made grouping C. magnificus C. sanguinea Calyptorhynchus Callocephalon C. leadbeateri and evolutionary relationships of cockatoos C. ophthalmia Brown & Toft (1999) based on morphological characters, isozyme, C. haematuropygia and mitochondrial DNA. However, their MATERIALS AND METHODS relationships are still controversial, especially Phylogeny based on different characters concerning the position of Nymphycus Cacatua leadbeateri Blood samples from each individual of 15 species, Biochemical 6 genera, and 3 tribes of cockatoos hollandicus. Since the nuclear β-fibrinogen (Adams et -

Society for Growing Australian Plants, Cairns Branch
Society for Growing Australian Plants, Cairns Branch Newsletter 147 March 2015 In this issue… EXCURSION REPORT – STONEY CREEK, FEBRUARY 2015 ............................... 1 ANNUAL GENERAL MEETING NOTIFICATION .. 4 EXCURSION REPORT (CONTINUED FROM PAGE 3).................................... 5 SPECIES LIST : STONEY CREEK BELOW FALLS AND RAILWAY ...................... 5 WHAT’S HAPPENING… ...... 9 CAIRNS SGAP ................ 9 TABLELANDS SGAP .......... 9 EXCURSION REPORT – STONEY CREEK , FEBRUARY 2015 TOWNSVILLE SGAP ......... 9 Boyd Lenne I arrived at Stoney Creek hopeful that one of our resident experts would be in attendance. Needless to say I was overjoyed to find Bob Jago unfolding his tea table for a pre walk cuppa. We were soon joined by Coralie Stuart and Anne Mohun. Anne and I had both come from a Treeforce planting event that morning, so we felt very intrepid to make the extra mission to a SGAP event all afternoon. In no time at all we four were huddled under a small interpretive shelter chatting, and playing "how many trees can Bob name while sitting down over smoko". The rain abated, and we were off. At the carpark I was interested in a striking prodigious orange fig with an attractive leaf and undulate leaf margin. Bob informed me this was the Ficus virgata, var. virgata . Trip trapping over the bridge, we saw the incredibly dainty Maesa haplobotrys , displaying fruit and flowers at all stages. Very attractive. The waters below were speckled with bright Syzygium luehmannii fruit. Up the hill, and water was playing across the path. Bob demonstrated the Rhodamnia spongiosa , with its incredibly pronounced three veined leaf. Myrtaceae are not particularly known for their three veined leaf structure, and I mused about whether many of the FNQ Lauraceae displayed this structural diagnostic. -

The Status and Impact of the Rainbow Lorikeet (Trichoglossus Haematodus Moluccanus) in South-West Western Australia
Research Library Miscellaneous Publications Research Publications 2005 The status and impact of the Rainbow lorikeet (Trichoglossus haematodus moluccanus) in south-west Western Australia Tamara Chapman Follow this and additional works at: https://researchlibrary.agric.wa.gov.au/misc_pbns Part of the Behavior and Ethology Commons, Biosecurity Commons, Environmental Studies Commons, Ornithology Commons, and the Population Biology Commons Recommended Citation Chapman, T. (2005), The status and impact of the Rainbow lorikeet (Trichoglossus haematodus moluccanus) in south-west Western Australia. Department of Primary Industries and Regional Development, Western Australia, Perth. Report 04/2005. This report is brought to you for free and open access by the Research Publications at Research Library. It has been accepted for inclusion in Miscellaneous Publications by an authorized administrator of Research Library. For more information, please contact [email protected]. ISSN 1447-4980 Miscellaneous Publication 04/2005 THE STATUS AND IMPACT OF THE RAINBOW LORIKEET (TRICHOGLOSSUS HAEMATODUS MOLUCCANUS) IN SOUTH-WEST WESTERN AUSTRALIA February 2005 © State of Western Australia, 2005. DISCLAIMER The Chief Executive Officer of the Department of Agriculture and the State of Western Australia accept no liability whatsoever by reason of negligence or otherwise arising from use or release of this information or any part of it. THE STATUS AND IMPACT OF THE RAINBOW LORIKEET (TRICHOGLOSSUS HAEMATODUS MOLUCCANUS) IN SOUTH-WEST WESTERN AUSTRALIA By Tamra -

Rose-Breasted Cockatoo (Galah Cockatoo) Eolophus Roseicapilla
Rose-breasted Cockatoo (Galah Cockatoo) Eolophus roseicapilla Class: Aves Order: Psittaciformes Family: Cacatuidae Characteristics: L. 35-38 cm; wt 300-435 gms. Females smaller. Gray back and flight feathers; pale pink crown; rose-red neck and underparts. Bone- colored beak; gray legs. Male: dark brown iris. Female: pink iris. Color of juveniles duller than adults. A highly intelligent, social and highly adaptable animal. Behavior: Bold and loud. Rely heavily on sense of sight. Highly social and long-lived. Bonded pairs have strong lifelong bonds with their partners. Preen facial feathers to show affection. Not highly territorial and often share roosting trees and food sources though minor squabbles frequently occur. Flocks congregate and forage on foot for food in open grassy areas. Communication consists of a high-pitched, splintered identifying call "chill chill; " harsher screeches when threatened, fighting or just having fun; and Range & Habitat: Mainland soft, muffled calls to initiate close contact. Australia and Tasmania in open habitats and urban areas such as Reproduction: Bonded pairs separate from flock and nest in tree cavities semi-desert, plains, open where a clutch of 2-5 white eggs is incubated 25 days by both parents. Fed with regurgitated food, chicks leave the nest about 49 days after hatching; woodland, farmlands and fields. reaching maturity in 4 years. Young have grayish plumage and a grey periophthalmic ring (naked area around their eyes) that fades as they approach maturity. Diet: Wild: grasses, herbs, seeds, nuts, berries, roots, green shoots, leaf buds, cereal crops, sunflower seeds; insects and larvae during breeding. Zoo: Cockatoo pellets, chopped fruit and vegetables, sunflower seeds (for training). -

Guidelines for Reducing Cockatoo Damage(PDF, 973.6
Guidelines for Reducing Cockatoo Damage Wildlife Management Methods Photo and Figure credits Cover photograph: Sulphur Crested Cockatoo – Nick Talbot Figure 1: Long-billed Corella – Drawing courtesy of Jess Davies Sulphur-crested Cockatoo and Galah – Drawings courtesy of Nic Day Figure 2: Kite to simulate bird of prey – Zoe Elliott Figure 3: Galah – Nick Talbot Figure 4: Long-billed Corella – Ian Temby Figure 5: Cockatoo damage to timber frames – Jim O’Brien Figure 6: Cockatoo damage to outdoor furniture – Ian Temby Figure 7: Cockatoo damage to sporting ground – Mark Breguet Figure 8: Corellas feeding on grain – Mark Breguet Figure 9: Cockatoo damage to crops – Ian Temby © The State of Victoria Department of Environment, Land, Water and Planning 2018 This work is licensed under a Creative Commons Attribution 4.0 International licence. You are free to re-use the work under that licence, on the condition that you credit the State of Victoria as author. The licence does not apply to any images, photographs or branding, including the Victorian Coat of Arms, the Victorian Government logo and the Department of Environment, Land, Water and Planning (DELWP) logo. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ ISBN 978-1-76047-876-6 pdf/online Disclaimer This publication may be of assistance to you but the State of Victoria and its employees do not guarantee that the publication is without flaw of any kind or is wholly appropriate for your particular purposes and therefore disclaims all liability for any error, loss or other consequence which may arise from you relying on any information in this publication. -

Australian Threatened Species: Carnaby's Black-Cockatoo
AustralianAustralian ThreatenedThreatened SpeciesSpecies Carnaby’s Black-Cockatoo Calyptorhynchus latirostris Conservation Status Commonwealth: Endangered (Environment Protection and Biodiversity Conservation Act 1999) WA: ‘Specially protected fauna’ (Western Australian Wildlife Conservation Act 1950) What do they look like? This large black cockatoo (also known as the Short-billed Black-Cockatoo) has white tail panels, white cheek patches and a short bill. It lives only in southwest Australia where large-scale clearing for farming has fragmented much of its habitat, particularly mature eucalypts such as salmon gum and wandoo that have suitable hollows for nesting. Where do they live? Carnaby’s Black-Cockatoo is endemic to southwest Western Australia, extending from the Murchison River to Esperance, and inland to Coroow, Kellerberrin and Lake Cronion. Most breeding occurs in areas with an average annual rainfall of 300-750mm, typically in the Wheatbelt and Great Southern regions. For nesting, Carnaby’s Black-Cockatoos require HowHow many areare there?there? eucalypt woodland, comprising principally of salmon gum or It is difficult to know how many Carnaby’s Black-Cockatoos wandoo. Their food is found in shrubland, or kwongan heath. are left, but it is known that their populations have declined by over 50% in the past 45 years, and that they no longer breed The cockatoos require a close association between breeding in up to a third of their former breeding sites in the Wheatbelt. and feeding sites during the breeding season. If these two very different habitats are not within a reasonable distance of each They are gregarious birds and live in pairs or small flocks during other, breeding attempts fail. -

The Avicultural Society of New South Wales Inc. (ASNSW) Black Cockatoos
The Avicultural Society of New South Wales Inc. (ASNSW) (Founded in 1940 as the Parrot & African Lovebird Society of Australia) Black Cockatoos (ASNSW The Avicultural Review - Volume 15 No. 3 April/May 1993) Among the most fascinating and majestic of our birds are the black cockatoos. The six species that fall into this descriptive group have colonised almost every area of Australia, adapting to a wide range of climates and landscapes. Few sights are more rewarding to the naturalist than seeing a party of these birds circling and wheeling high in the air, before descending on a stand of eucalypts or casuarinas. Introduction The black cockatoos are divided into three genera — Probisciger, Calyptorhynchus and Callocephalon. All are characterised by a dark or black body, strong beak and legs and feet well adapted for gripping. Nesting is carried out high in a tree, in a hollow limb, where one or two eggs are laid. Incubation is undertaken by the female, who is fed by the male during her time at the nest. The young, when they hatch, are naked and helpless, and will stay in the nest for about 10-12 weeks before venturing into the outside world. Large numbers of black cockatoos were taken for the pet trade before controls were introduced. Generally, the young were removed from the nest and raised by hand. If the nest was inaccessible, then the whole tree was cut down — a practice which effectively diminished the supply of nesting sites for future seasons. Today, the black cockatoos are fully protected, but destruction of habitat is still a threat as more areas are cleared for agriculture. -

Field River and Glenthorne Farm Ground to Forage for Food
YELLOW-RUMPED THORNBILL The Yellow-rumped Thornbill is a small insect eating bird which ocassionally eats seeds. It associates in small flocks, often flying from small shrubs down onto the Field River and Glenthorne Farm ground to forage for food. When disturbed, these birds alight back into the shrubs, revealing their bright yellow rumps. Seen in the open fields along the Field River, these birds will adapt to living close to suburbs provided adequate open space is Bird List preserved for them. They live in woodlands but can happily survive around mown The local open space of the southern suburbs is an important corridor linking the Mount Lofty Ranges to the sea. fields if sufficient native habitat remains. They have been seen in reasonable The variety of landscapes within this area provides ideal habitat for the varying needs of many different birds numbers near Hugh Johnson Reserve, Sheidow Park and along the Southern and because of this the opportunity exists to see many Australian birds close to our suburban homes. Expressway near Trott Park. We hope that when you are out walking, this bird list may assist you to identify some of the birds you see. YELLOW-TAILED BLACK COCKATOO Yellow-tailed Black Cockatoos are frequent visitors to the southern suburbs, looking for pine nuts in local trees after the breeding season has been completed elsewhere. These birds congregate in large flocks and are seen at certain times of the year in Reynella, in Sheidow Park and in 2007 about 200 appeared at Glenthorne Farm one Sunday morning as the Friends of Glenthorne were working. -
Bird Guide for the Great Western Woodlands Male Gilbert’S Whistler: Chris Tzaros Whistler: Male Gilbert’S
Bird Guide for the Great Western Woodlands Male Gilbert’s Whistler: Chris Tzaros Whistler: Male Gilbert’s Western Australia PART 1. GWW NORTHERN Southern Cross Kalgoorlie Widgiemooltha birds are in our nature ® Australia AUSTRALIA Introduction The birds and places of the north-west region of the Great Western Woodlands are presented in this booklet. This area includes tall woodlands on red soils, shrublands on yellow sand plains and mallee on sand and loam soils. Landforms include large granite outcrops, Banded Ironstone Formation (BIF) Ranges, extensive natural salt lakes and a few freshwater lakes. The Great Western Woodlands At 16 million hectares, the Great Western Woodlands (GWW) is close to three quarters the size of Victoria and is the largest remaining intact area of temperate woodland in the world. It is located between the Western Australian Wheatbelt and the Nullarbor Plain. BirdLife Australia and The Nature Conservancy joined forces in 2012 to establish a long-term project to study the birds of this unique region and to determine how we can best conserve the woodland birds that occur here. Kalgoorlie 1 Groups of volunteers carry out bird surveys each year in spring and autumn to find out the species present, their abundance and to observe their behaviour. If you would like to know more visit http://www.birdlife.org.au/projects/great-western-woodlands If you would like to participate as a volunteer contact [email protected]. All levels of experience are welcome. The following six pages present 48 bird species that typically occur in four different habitats of the north-west region of the GWW, although they are not restricted to these. -

Rose-Breasted Cockatoos Eolophis Roseicapillus by Jim C
Rose-breasted Cockatoos Eolophis roseicapillus By Jim C. Hawley Jr., EA • Queen Creek, Arizona Rose-breasted cockatoos are among the most beautiful is translated in my opinion to over feeding. The of all the parrots; Looked after properly, treated with Rose-breasted cockatoo has evolved into a very the best of care and fed the proper diets, they can result finely tuned feast or famine survivor. In their native in the most prolific breeders that you might ever have in your aviaries. Handfed Rose-breasted cockatoos are habitat, they are accustomed to abundant times not only good breeders, but they make delightful pets of food availability and devastating drought con- as well. ditions at other times. Their metabolisms have developed the ability to store fat in reserve during Captive Breeding abundant feed and to draw on these during fam- ine. Of course this is accompanied with strenuous There are many opinions and ideas that people exercise throughout the year flying to and from share from time to time claiming to be “The” one food sources, nesting sights and courtship. Not to and only method of success for keeping and rais- mention the rearing of young in-between all of this ing Rose-breasted cockatoos or Galahs, as they activity. are known in their native Australia. One of my favorite was one opinion shared with me, when In captivity we have a tendency to feed fattening Joseph Forshaw was visiting our farm. He asked seed diets to our parrots and house them in low why I kept Galahs. (As most of you are aware, the activity tolerant caging.