Scaevola Taccada (Gaertn.) Roxb
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Boophone Disticha
Micropropagation and pharmacological evaluation of Boophone disticha Lee Cheesman Submitted in fulfilment of the academic requirements for the degree of Doctor of Philosophy Research Centre for Plant Growth and Development School of Life Sciences University of KwaZulu-Natal, Pietermaritzburg April 2013 COLLEGE OF AGRICULTURE, ENGINEERING AND SCIENCES DECLARATION 1 – PLAGIARISM I, LEE CHEESMAN Student Number: 203502173 declare that: 1. The research contained in this thesis, except where otherwise indicated, is my original research. 2. This thesis has not been submitted for any degree or examination at any other University. 3. This thesis does not contain other persons’ data, pictures, graphs or other information, unless specifically acknowledged as being sourced from other persons. 4. This thesis does not contain other persons’ writing, unless specifically acknowledged as being sourced from other researchers. Where other written sources have been quoted, then: a. Their words have been re-written but the general information attributed to them has been referenced. b. Where their exact words have been used, then their writing has been placed in italics and inside quotation marks, and referenced. 5. This thesis does not contain text, graphics or tables copied and pasted from the internet, unless specifically acknowledged, and the source being detailed in the thesis and in the reference section. Signed at………………………………....on the.....….. day of ……......……….2013 ______________________________ SIGNATURE i STUDENT DECLARATION Micropropagation and pharmacological evaluation of Boophone disticha I, LEE CHEESMAN Student Number: 203502173 declare that: 1. The research reported in this dissertation, except where otherwise indicated is the result of my own endeavours in the Research Centre for Plant Growth and Development, School of Life Sciences, University of KwaZulu-Natal, Pietermaritzburg. -

A Refugium for Scaevola Plumieri, a Highly Threatened Rare Plant in Sri Lanka
Cey. J. Sci. (Bio. Sci.) Vol 34, 2005, 21-25 21 A REFUGIUM FOR SCAEVOLA PLUMIERI, A HIGHLY THREATENED RARE PLANT IN SRI LANKA A.H.Magdon Jayasuriya Environment & Management Lanka, 68 Davidson Road, Colombo 4, Sri Lanka ABSTRACT Scaevola plumieri ( “Hin-takkada”), a surface-creeping shrub among strand vegetation, is extremely rare and “highly threatened” having been previously recorded from no more than six locations in Sri Lanka, mostly during the 19th century. The species is presumed to be extinct in its 19th century localities on the west and east coasts due to loss of habitat, and in its 20th century localities on the east coast due to the recent Tsunami disaster. The Puttalam lagoon shore within the Wilpattu National Park, where this plant was recently located, will therefore be a refugium for this species. Incidentally, this is also the first time that this plant was observed on a lagoon-shore habitat in Sri Lanka, instead of its usual sea shore habitat. This indicates the high conservation value of the Wilpattu beach front of the Kala Oya Lower Basin which also harbours one of the last remaining pristine stretches of mangroves in Sri Lanka, which should be noted by the Department of Wildlife Conservation in park planning and management. INTRODUCTION Scaevola plumieri (L.) Vahl belongs to the activities in coastal zones, “ Hin-takkada” populations have presumably disappeared from the family Goodeniaceae and its local name is th (“Hin-takkada”). It is a surface-creeping above localities. The specimens recorded in the 20 (prostate) shrub with rounded and leathery century were gathered between 1969 – 1974 only (coriaceaous) leaves not exceeding 8 cm in from two localities on the lower east coast, namely length, while the fruit (drupe) is red when ripe. -

Last Frontiers-Belize
Last Frontiers-Belize (I) Gales Point – Belize District - Belize Quick identification guide for some conspicuous or common native vegetation Photos © Jan Meerman, Belize Tropical Forest Studies, 2010 Typha dominguensis Bulbostylis sp. Rhynchospora barbata Fibristylis sp. Acrostychum aureum Typhaceae Cyperaceae Cyperaceae Cyperaceae Pteridophyta Reed: Wetland Sedge: Savanna Sedge: Savanna Sedge: Savanna Fern: Wetland Zamia prasina Zamia meermanii Acoelorraphe wrightii Attalea cohune Attalea cohune Zamiaceae Zamiaceae Arecaeae Arecaceae Arecaceae Cycad: Savanna/Forest Cycad: Steep Cliffs Palm: Wet areas Palm: Hills Palm: Hills Schippia concolor Bactris mexicana Bactris mexicana Brassavola nodosa Oeceoclades maculata Arecaceae Arecaceae Palm: Arecaceae Orchidaceae Orchidaceae Palm: Savanna scrub Wet forest Palm: Wet forest Orchid: Savanna Orchid: Hill forest Catasetum Vriesea heliconoides. Catopsis berteroana Najas wrightii Xyris ambigua integerrimum Bromeliaceae Bromeliaceae Najadaceae Xyridaceae Orchidaeceae Bromeliad: on Trees in Bromeliad: on trees Aquatic plant: Herb: Savanna Orchid: On Trees wet forest wetlands Last Frontiers-Belize (II) Gales Point – Belize District - Belize Quick identification guide for some conspicuous or common native vegetation Photos © Jan Meerman, Belize Tropical Forest Studies, 2010 Anisantherina hispidula Buchnera pusilla Dalechampia schippii Hypericum terra-firmae Polygala sp. Polygalaceae Scrophulariaceae. Herb: Scrophulariaceae Euphorbiaceae Vine: Clusiaceae Herb: Herb: Savanna Savanna Herb: Savanna Savanna -

Dermatitis Por Contacto a Metopium Brownei (Chechem). Observaciones Clínicas De 20 Casos En Quintana Roo, México Contact Dermatitis Caused by Metopium Brownei
Artículos Originales DermatologíaCMQ2009;7(4):226-233 Dermatitis por contacto a Metopium brownei (Chechem). Observaciones clínicas de 20 casos en Quintana Roo, México Contact dermatitis caused by Metopium brownei. Report of 20 cases in Quintana Roo, Mexico Marco Romano Quintanilla*, Roberto Arenas** * Dermatólogo. Clínica Carranza. Venustiano Carranza 366. Chetumal Q. Roo , México [email protected] ** Jefe de la Sección de Micología. Departamento de Dermatología Hospital General Fecha de aceptación: Agosto 2009 Resumen NTECEDENTES: Las fitodermatitis son frecuentes en la práctica dermatológica. En la península de Yucatán observamos entre otras, la ocasionada por METOPIUM BROWNEI cuyo componente tóxico es el urushiol. No hay Acomunicaciones previas de esta dermatosis en la literatura revisada. OBJETIVO: Identificar las principales características clínicas de la dermatitis reaccional por METOPIUM BROWNEI. METODOLOGÍA: Estudio retrospectivo de 20 casos. Se registraron las siguientes variables: edad, sexo, ocupación, sitio de exposición, tiempo de aparición de las primeras manifestaciones, topografía, morfología y diagnóstico clínico. No se realizaron pruebas epicutáneas. RESULTADOS: 65% fueron de sexo masculino, con un promedio de edad de 25.1 años. No se documentó una ocupación predominante, la selva fue el sitio de encuentro con el árbol (90%) y el tiempo de inicio de las manifestaciones fue en promedio, de 2.5 días luego de la exposición. Las reacciones tipo eccema fueron las más comunes. En ausencia de pruebas de parche, se realizó el diagnóstico clínico probable de dermatitis por contacto alérgica en 9 de los 20 pacientes (45%), en 30% tanto irritativa como alérgica, en 1% fotosensibilidad y en 1% dermatitis por contacto aerotransportada. CONCLUSIONES: Las reacciones a METOPIUM BROWNEI corresponden principalmente a un probable proceso alérgico local, sistémico, con fotosensibilidad o aerotransportado. -

Island Biology Island Biology
IIssllaanndd bbiioollooggyy Allan Sørensen Allan Timmermann, Ana Maria Martín González Camilla Hansen Camille Kruch Dorte Jensen Eva Grøndahl, Franziska Petra Popko, Grete Fogtmann Jensen, Gudny Asgeirsdottir, Hubertus Heinicke, Jan Nikkelborg, Janne Thirstrup, Karin T. Clausen, Karina Mikkelsen, Katrine Meisner, Kent Olsen, Kristina Boros, Linn Kathrin Øverland, Lucía de la Guardia, Marie S. Hoelgaard, Melissa Wetter Mikkel Sørensen, Morten Ravn Knudsen, Pedro Finamore, Petr Klimes, Rasmus Højer Jensen, Tenna Boye Tine Biedenweg AARHUS UNIVERSITY 2005/ESSAYS IN EVOLUTIONARY ECOLOGY Teachers: Bodil K. Ehlers, Tanja Ingversen, Dave Parker, MIchael Warrer Larsen, Yoko L. Dupont & Jens M. Olesen 1 C o n t e n t s Atlantic Ocean Islands Faroe Islands Kent Olsen 4 Shetland Islands Janne Thirstrup 10 Svalbard Linn Kathrin Øverland 14 Greenland Eva Grøndahl 18 Azores Tenna Boye 22 St. Helena Pedro Finamore 25 Falkland Islands Kristina Boros 29 Cape Verde Islands Allan Sørensen 32 Tristan da Cunha Rasmus Højer Jensen 36 Mediterranean Islands Corsica Camille Kruch 39 Cyprus Tine Biedenweg 42 Indian Ocean Islands Socotra Mikkel Sørensen 47 Zanzibar Karina Mikkelsen 50 Maldives Allan Timmermann 54 Krakatau Camilla Hansen 57 Bali and Lombok Grete Fogtmann Jensen 61 Pacific Islands New Guinea Lucía de la Guardia 66 2 Solomon Islands Karin T. Clausen 70 New Caledonia Franziska Petra Popko 74 Samoa Morten Ravn Knudsen 77 Tasmania Jan Nikkelborg 81 Fiji Melissa Wetter 84 New Zealand Marie S. Hoelgaard 87 Pitcairn Katrine Meisner 91 Juan Fernandéz Islands Gudny Asgeirsdottir 95 Hawaiian Islands Petr Klimes 97 Galápagos Islands Dorthe Jensen 102 Caribbean Islands Cuba Hubertus Heinicke 107 Dominica Ana Maria Martin Gonzalez 110 Essay localities 3 The Faroe Islands Kent Olsen Introduction The Faroe Islands is a treeless archipelago situated in the heart of the warm North Atlantic Current on the Wyville Thompson Ridge between 61°20’ and 62°24’ N and between 6°15’ and 7°41’ W. -

The Origin of the Bifurcating Style in Asteraceae (Compositae)
Annals of Botany 117: 1009–1021, 2016 doi:10.1093/aob/mcw033, available online at www.aob.oxfordjournals.org The origin of the bifurcating style in Asteraceae (Compositae) Liliana Katinas1,2,*, Marcelo P. Hernandez 2, Ana M. Arambarri2 and Vicki A. Funk3 1Division Plantas Vasculares, Museo de La Plata, La Plata, Argentina, 2Laboratorio de Morfologıa Comparada de Espermatofitas (LAMCE), Facultad de Ciencias Agrarias y Forestales, Universidad Nacional de La Plata, La Plata, Argentina and 3Department of Botany, NMNH, Smithsonian Institution, Washington D.C., USA *For correspondence. E-mail [email protected] Received: 20 November 2015 Returned for revision: 22 December 2015 Accepted: 8 January 2016 Published electronically: 20 April 2016 Background and Aims The plant family Asteraceae (Compositae) exhibits remarkable morphological variation in the styles of its members. Lack of studies on the styles of the sister families to Asteraceae, Goodeniaceae and Calyceraceae, obscures our understanding of the origin and evolution of this reproductive feature in these groups. The aim of this work was to perform a comparative study of style morphology and to discuss the relevance of im- portant features in the evolution of Asteraceae and its sister families. Methods The histochemistry, venation and general morphology of the styles of members of Goodeniaceae, Calyceraceae and early branching lineages of Asteraceae were analysed and put in a phylogenetic framework to dis- cuss the relevance of style features in the evolution of these families. Key Results The location of lipophilic substances allowed differentiation of receptive from non-receptive style papillae, and the style venation in Goodeniaceae and Calyceraceae proved to be distinctive. -

Pacific Islands Area
Habitat Planting for Pollinators Pacific Islands Area November 2014 The Xerces Society for Invertebrate Conservation www.xerces.org Acknowledgements This document is the result of collaboration with state and federal agencies and educational institutions. The authors would like to express their sincere gratitude for the technical assistance and time spent suggesting, advising, reviewing, and editing. In particular, we would like to thank the staff at the Hoolehua Plant Materials Center on the Hawaiian Island of Molokai, NRCS staff in Hawaii and American Samoa, and researchers and extension personnel at American Samoa Community College Land Grant (especially Mark Schmaedick). Authors Written by Jolie Goldenetz-Dollar (American Samoa Community College), Brianna Borders, Eric Lee- Mäder, and Mace Vaughan (The Xerces Society for Invertebrate Conservation), and Gregory Koob, Kawika Duvauchelle, and Glenn Sakamoto (USDA Natural Resources Conservation Service). Editing and layout Ashley Minnerath (The Xerces Society). Updated November 2014 by Sara Morris, Emily Krafft, and Anne Stine (The Xerces Society). Photographs We thank the photographers who generously allowed use of their images. Copyright of all photographs remains with the photographers. Cover main: Jolie Goldenetz-Dollar, American Samoa Community College. Cover bottom left: John Kaia, Lahaina Photography. Cover bottom right: Gregory Koob, Hawaii Natural Resources Conservation Service. Funding This technical note was funded by the U.S. Department of Agriculture (USDA) Natural Resources Conservation Service (NRCS) and produced jointly by the NRCS and The Xerces Society for Invertebrate Conservation. Additional support was provided by the National Institute for Food and Agriculture (USDA). Please contact Tony Ingersoll ([email protected]) for more information about this publication. -

New Hawaiian Plant Records from Herbarium Pacificum for 20081
Records of the Hawaii Biological Survey for 2008. Edited by Neal L. Evenhuis & Lucius G. Eldredge. Bishop Museum Occasional Papers 107: 19–26 (2010) New Hawaiian plant records from Herbarium Pacificum for 2008 1 BARBARA H. K ENNEDY , S HELLEY A. J AMES , & CLYDE T. I MADA (Hawaii Biological Survey, Bishop Museum, 1525 Bernice St, Honolulu, Hawai‘i 96817-2704, USA; emails: [email protected], [email protected], [email protected]) These previously unpublished Hawaiian plant records report 2 new naturalized records, 13 new island records, 1 adventive species showing signs of naturalization, and nomen - clatural changes affecting the flora of Hawai‘i. All identifications were made by the authors, except where noted in the acknowledgments, and all supporting voucher speci - mens are on deposit at BISH. Apocynaceae Rauvolfia vomitoria Afzel. New naturalized record The following report is paraphrased from Melora K. Purell, Coordinator of the Kohala Watershed Partnership on the Big Island, who sent an email alert to the conservation com - munity in August 2008 reporting on the incipient outbreak of R. vomitoria, poison devil’s- pepper or swizzle stick, on 800–1200 ha (2000–3000 acres) in North Kohala, Hawai‘i Island. First noticed by field workers in North Kohala about ten years ago, swizzle stick has become a growing concern within the past year, as the tree has spread rapidly and invaded pastures, gulches, and closed-canopy alien and mixed alien-‘ōhi‘a forest in North Kohala, where it grows under the canopies of eucalyptus, strawberry guava, common guava, kukui, albizia, and ‘ōhi‘a. The current distribution is from 180–490 m (600–1600 ft) elevation, from Makapala to ‘Iole. -

Phyllosticta Hymenocallidicola Fungal Planet Description Sheets 139
138 Persoonia – Volume 27, 2011 Phyllosticta hymenocallidicola Fungal Planet description sheets 139 Fungal Planet 95 – 6 December 2011 Phyllosticta hymenocallidicola Crous, Summerell & Romberg, sp. nov. Phyllostictae citricarpae similis, sed conidiis minoribus, (8–)9–10(–11) × Typus. AUSTRALIA, Northern Territory, Darwin, Charles Darwin University, (6–)6.5–7 µm, discernitur. S 12°22'25.2" E 130°52'07.4" on leaves of Hymenocallis littoralis (Amaryl lidaceae), 1 May 2011, P.W. Crous & M. Romberg, holotype CBS H-20759, Etymology. Named after the host genus from which it was isolated, cultures ex-type CPC 19332, 19331 = CBS 131309, ITS sequence GenBank Hymenocallis. JQ044423 and LSU sequence GenBank JQ044443, MycoBank MB560699; Associated with brown leaf spots and leaf tip blight. Conidio Darwin, in front of Vibe Hotel, Kitchener Drive, Darwin Conference Centre, on leaves of Hymenocallis littoralis, 27 Apr. 2011, P.W. Crous & B.A. Summerell, mata pycnidial, solitary, black, erumpent, globose, exuding CBS H-20760, cultures CPC 19330, 19329 = CBS 131310, ITS sequence colourless to opaque conidial masses; pycnidia up to 200 µm GenBank JQ044424. diam; pycnidial wall of several layers of brown textura angula ris, up to 30 µm thick; inner layer of hyaline textura angularis. Notes — During a meeting of the Australasian Society for Ostiole central, up to 20 µm diam, rim lined with darker brown Plant Pathology in Darwin (April 2011), a serious leaf spot and cells. Conidiophores subcylindrical to ampulliform, reduced tip blight disease was noticed on the Hymenocallis littoralis to conidiogenous cells, or with 1–2 supporting cells, at times plants growing in front of the conference centre. -
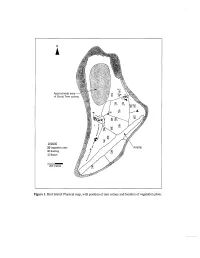
Figure 1. Bird Island: Physical Map, with Position of Tern Colony and Location of Vegetation Plots
Approximate of Sooty Ter~ Leqend (XI Vegetat~onplots 9 Building 6 Beach 200 metres Figure 1. Bird Island: Physical map, with position of tern colony and location of vegetation plots. BIRD MICHAEL J. HILL', TERENCE M. VEL', KATHRYN J. HOLM^, STEVEN J. PARR~ and NIRMAL J. SHAH' GEOLOGY, TOPOGRAPHY AND CLIMATE Bird is the northernmost island of the Seychelles, lying around 90 km north of Mahe, the largest of the granitic Seychelles, at the northern edge of the Seychelles bank. Different published sources vary in the estimated area of Bird Island with figures of c. 70 ha given by Feare (1979), 82 ha in Stoddart and Fosberg (1981), 101 ha in Skerrett et al. (2001), and 120.7 ha from recent aerial photographs (Ministry of Land Use and Habitat, Seychelles, unpublished data). In part, this variation may be explained by seasonal or longer-term variations in the vegetated area of the island; Bird Island is relatively dynamic, experiencing considerable coastal changes over time (Feare, 1979). The maximum elevation is less than 4 m above sea level. Unlike the majority of islands on the Seychelles Bank, Bird has no exposed granite and it is entirely formed of reef-derived sands. The accumulation of guano on sand deposits has led to the formation of phosphatic sandstone over 26% of the island's surface (Baker, 1963). Phosphatic sandstone is concentrated in a central band; the island's coastal zone is entirely sandy. Most of the original guano has now been removed for export. The soils of Bird Island are of two main series; over the central phosphatic sandstone area, Jemo series soils (missing their upper layer of guano) occur. -

Common Plants of the Maldives Common Plants Common Plants of the Maldives Is a Starting Point for People Interested in Learning About Trees and Shrubs of the Maldives
series 1 series 1 Common plants of the Maldives Common plants Common Plants of the Maldives is a starting point for people interested in learning about trees and shrubs of the Maldives. It contains of the Maldives descriptions and photographs to help identify local plants as well as information on traditional uses in the Maldives and throughout the world. Whether you’re relaxing in your deck-chair or exploring the island vegetation, you will come to learn that all plants, within every ecosystem are not only beautiful but important for our survival as they provide food, medicine, soil stability, fresh air and water. books in this series are: Common Plants of the Maldives, Common Birds of the Maldives and Life on the Beach, Maldives. series 1 series 1 series 1 Common plants Common birds life on the beach of the Maldives of the Maldives Maldives LIVE&LEARN Environmental Education www.livelearn.org Common plants of the Maldives LIVE&LEARN Environmental Education Haa Alifu Atoll Haa Dhaalu INDIAN OCEAN The Maldives Atoll m There are Shaviyani Atoll approximately 1190 islands in the Maldives with some Noonu Atoll form of vegetation on Raa Atoll them. Lhaviyani Atoll m Approximately 200 are inhabited Baa Atoll islands and 990 are uninhabited. m There are 26 distinct Kaafu Atoll (Malé Atoll) geographical atolls. Alifu Alifu Atoll These are divided MALÉ into 20 administrative regions, with the Alifu Dhaalu Atoll capital Male’ making up a separate Vaavu Atoll administrative unit. Faafu Atoll m The Maldives is 860km long and Meemu Atoll 130km wide. Dhaalu Atoll m More than 99% of the country is water (115,000km2) with Thaa Atoll less than 0.3% land (300km2). -

Fleurs Et Arbres Des Seychelles
fleurs et arbres r~ des c§l#-~ SEYCHELLES OUVRAGE EDITE POUR LE COMPTE DU DEPARTEMENT DES FINANCES DES SEYCHELLES © 1986 Francis Friedmann et Département des Finances des Seychelles Toute reproduction, même partielle, par quelque procédé que ce soit, est rigoureusement interdite sans accord préalable de "Auteur et du Département des Finances des Seychelles, 2 fleurs et arbres r"~ des @/?-.... SEYCHELLES Texte et photos FRANCIS FRIEDMANN Office de la Recherche Scientifique et Technique Outre-Mer. (Pari~RSTOM Fonds Ducumt:llt ire N° ~ ~ O~ g rz. .0 o9 NOV. 1990 Cote e .-""----........... KENYA ,'" ...... " " .. " " .. 1" . 1 •• e. 1 1 ... 1 • • 1 ... 1 • 1 1 •• 1 • • MAHE " :. .,-.,-----.. ... " " • • L.LI ,'" ---- • Z " • « ZONE MARITIME ECONOMIQUE • N <," EXCLUSIVE DES SEYCHELLES Z \ « \\ 1- \\,.ALDABRA .. (",-------- "'" ••• 1 ,",. 1 , ...... " " 1 .. ',_--- --.... , ", :1 ~ ......... 1 ...... 1 4:) 0 ...... ) L.LI ...................' ::::l COMORES 0 o al ::E« No ::E REPUBLIQUE DES SEYCHELLES 4 .... ......, " \ "\ \ 1 1 1 1 ,1 , . ,, \ \ \ \ ,,\ ,, --------' OCEAN INDIEN Archipel central <:> ILE AUX VACHES DENIS ARIDE PETITE c::a SOEUR GRANDE SOEUR CURIEUSE ~ C> 0 ~ OFELICITE PRASLlN~ D tJMARIANNE ILE NORD ~ LA DIGUE <)SILHOUETTE ~FREGATE 5 PREFACE Les mers ~haudes, les plages dorées de sable fin, les récifs de coraux se retrouvent dans de nombreux pays sous les tropiques. Mais aucun d'entre eux ne peut se prévaloir à la fois d'une aussi grande richesse en plantes. oiseaux et animaux d'espèces rares, et d'un accueil aussi chaleureux que les Seychelles. Cet accueil se traduit tout aussi bien par la flore et la faune; le gouverne ment a fait preuve d'une initiative de pionnier en déclarant l'Océan Indien Zone Protégée en ce qui concerne les baleines et les dauphins.