Phyllosticta Hymenocallidicola Fungal Planet Description Sheets 139
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Boophone Disticha
Micropropagation and pharmacological evaluation of Boophone disticha Lee Cheesman Submitted in fulfilment of the academic requirements for the degree of Doctor of Philosophy Research Centre for Plant Growth and Development School of Life Sciences University of KwaZulu-Natal, Pietermaritzburg April 2013 COLLEGE OF AGRICULTURE, ENGINEERING AND SCIENCES DECLARATION 1 – PLAGIARISM I, LEE CHEESMAN Student Number: 203502173 declare that: 1. The research contained in this thesis, except where otherwise indicated, is my original research. 2. This thesis has not been submitted for any degree or examination at any other University. 3. This thesis does not contain other persons’ data, pictures, graphs or other information, unless specifically acknowledged as being sourced from other persons. 4. This thesis does not contain other persons’ writing, unless specifically acknowledged as being sourced from other researchers. Where other written sources have been quoted, then: a. Their words have been re-written but the general information attributed to them has been referenced. b. Where their exact words have been used, then their writing has been placed in italics and inside quotation marks, and referenced. 5. This thesis does not contain text, graphics or tables copied and pasted from the internet, unless specifically acknowledged, and the source being detailed in the thesis and in the reference section. Signed at………………………………....on the.....….. day of ……......……….2013 ______________________________ SIGNATURE i STUDENT DECLARATION Micropropagation and pharmacological evaluation of Boophone disticha I, LEE CHEESMAN Student Number: 203502173 declare that: 1. The research reported in this dissertation, except where otherwise indicated is the result of my own endeavours in the Research Centre for Plant Growth and Development, School of Life Sciences, University of KwaZulu-Natal, Pietermaritzburg. -

Scaevola Taccada (Gaertn.) Roxb
BioInvasions Records (2021) Volume 10, Issue 2: 425–435 CORRECTED PROOF Rapid Communication First record of naturalization of Scaevola taccada (Gaertn.) Roxb. (Goodeniaceae) in southeastern Mexico Gonzalo Castillo-Campos1,*, José G. García-Franco2 and M. Luisa Martínez2 1Red de Biodiversidad y Sistemática, Instituto de Ecología, A.C., Xalapa, Veracruz, 91073, México 2Red de Ecología Funcional, Instituto de Ecología, A.C. Xalapa, Veracruz, 91073, México Author e-mails: [email protected] (GCC), [email protected] (JGGF), [email protected] (MLM) *Corresponding author Citation: Castillo-Campos G, García- Franco JG, Martínez ML (2021) First Abstract record of naturalization of Scaevola taccada (Gaertn.) Roxb. (Goodeniaceae) Scaevola taccada (Gaertn.) Roxb. is native of Asia and eastern Africa but has been in southeastern Mexico. BioInvasions introduced into the Americas as an ornamental urban plant. This paper reports, for Records 10(2): 425–435, https://doi.org/10. the first time, the presence of Scaevola taccada in natural environments from 3391/bir.2021.10.2.21 southeastern Mexico. Several populations of S. taccada were identified during a Received: 23 July 2020 botanical survey of the coastal dunes of the Cozumel Island Biosphere Reserve Accepted: 22 October 2020 (State of Quintana Roo, Mexico) aimed at recording the most common plant Published: 22 January 2021 species. Scaevola taccada is considered as an invasive species of coastal areas in this region. Evidence of its invasiveness is suggested by the fact that populations Handling editor: Oliver Pescott consisting of individuals of different size classes are found distributed throughout Thematic editor: Stelios Katsanevakis the island. Furthermore, they appear to belong to different generations since we Copyright: © Castillo-Campos et al. -
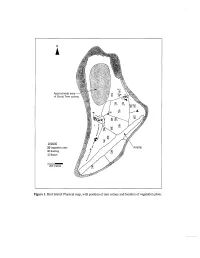
Figure 1. Bird Island: Physical Map, with Position of Tern Colony and Location of Vegetation Plots
Approximate of Sooty Ter~ Leqend (XI Vegetat~onplots 9 Building 6 Beach 200 metres Figure 1. Bird Island: Physical map, with position of tern colony and location of vegetation plots. BIRD MICHAEL J. HILL', TERENCE M. VEL', KATHRYN J. HOLM^, STEVEN J. PARR~ and NIRMAL J. SHAH' GEOLOGY, TOPOGRAPHY AND CLIMATE Bird is the northernmost island of the Seychelles, lying around 90 km north of Mahe, the largest of the granitic Seychelles, at the northern edge of the Seychelles bank. Different published sources vary in the estimated area of Bird Island with figures of c. 70 ha given by Feare (1979), 82 ha in Stoddart and Fosberg (1981), 101 ha in Skerrett et al. (2001), and 120.7 ha from recent aerial photographs (Ministry of Land Use and Habitat, Seychelles, unpublished data). In part, this variation may be explained by seasonal or longer-term variations in the vegetated area of the island; Bird Island is relatively dynamic, experiencing considerable coastal changes over time (Feare, 1979). The maximum elevation is less than 4 m above sea level. Unlike the majority of islands on the Seychelles Bank, Bird has no exposed granite and it is entirely formed of reef-derived sands. The accumulation of guano on sand deposits has led to the formation of phosphatic sandstone over 26% of the island's surface (Baker, 1963). Phosphatic sandstone is concentrated in a central band; the island's coastal zone is entirely sandy. Most of the original guano has now been removed for export. The soils of Bird Island are of two main series; over the central phosphatic sandstone area, Jemo series soils (missing their upper layer of guano) occur. -

Fleurs Et Arbres Des Seychelles
fleurs et arbres r~ des c§l#-~ SEYCHELLES OUVRAGE EDITE POUR LE COMPTE DU DEPARTEMENT DES FINANCES DES SEYCHELLES © 1986 Francis Friedmann et Département des Finances des Seychelles Toute reproduction, même partielle, par quelque procédé que ce soit, est rigoureusement interdite sans accord préalable de "Auteur et du Département des Finances des Seychelles, 2 fleurs et arbres r"~ des @/?-.... SEYCHELLES Texte et photos FRANCIS FRIEDMANN Office de la Recherche Scientifique et Technique Outre-Mer. (Pari~RSTOM Fonds Ducumt:llt ire N° ~ ~ O~ g rz. .0 o9 NOV. 1990 Cote e .-""----........... KENYA ,'" ...... " " .. " " .. 1" . 1 •• e. 1 1 ... 1 • • 1 ... 1 • 1 1 •• 1 • • MAHE " :. .,-.,-----.. ... " " • • L.LI ,'" ---- • Z " • « ZONE MARITIME ECONOMIQUE • N <," EXCLUSIVE DES SEYCHELLES Z \ « \\ 1- \\,.ALDABRA .. (",-------- "'" ••• 1 ,",. 1 , ...... " " 1 .. ',_--- --.... , ", :1 ~ ......... 1 ...... 1 4:) 0 ...... ) L.LI ...................' ::::l COMORES 0 o al ::E« No ::E REPUBLIQUE DES SEYCHELLES 4 .... ......, " \ "\ \ 1 1 1 1 ,1 , . ,, \ \ \ \ ,,\ ,, --------' OCEAN INDIEN Archipel central <:> ILE AUX VACHES DENIS ARIDE PETITE c::a SOEUR GRANDE SOEUR CURIEUSE ~ C> 0 ~ OFELICITE PRASLlN~ D tJMARIANNE ILE NORD ~ LA DIGUE <)SILHOUETTE ~FREGATE 5 PREFACE Les mers ~haudes, les plages dorées de sable fin, les récifs de coraux se retrouvent dans de nombreux pays sous les tropiques. Mais aucun d'entre eux ne peut se prévaloir à la fois d'une aussi grande richesse en plantes. oiseaux et animaux d'espèces rares, et d'un accueil aussi chaleureux que les Seychelles. Cet accueil se traduit tout aussi bien par la flore et la faune; le gouverne ment a fait preuve d'une initiative de pionnier en déclarant l'Océan Indien Zone Protégée en ce qui concerne les baleines et les dauphins. -

Atoll Research Bulletin No. 503 the Vascular Plants Of
ATOLL RESEARCH BULLETIN NO. 503 THE VASCULAR PLANTS OF MAJURO ATOLL, REPUBLIC OF THE MARSHALL ISLANDS BY NANCY VANDER VELDE ISSUED BY NATIONAL MUSEUM OF NATURAL HISTORY SMITHSONIAN INSTITUTION WASHINGTON, D.C., U.S.A. AUGUST 2003 Uliga Figure 1. Majuro Atoll THE VASCULAR PLANTS OF MAJURO ATOLL, REPUBLIC OF THE MARSHALL ISLANDS ABSTRACT Majuro Atoll has been a center of activity for the Marshall Islands since 1944 and is now the major population center and port of entry for the country. Previous to the accompanying study, no thorough documentation has been made of the vascular plants of Majuro Atoll. There were only reports that were either part of much larger discussions on the entire Micronesian region or the Marshall Islands as a whole, and were of a very limited scope. Previous reports by Fosberg, Sachet & Oliver (1979, 1982, 1987) presented only 115 vascular plants on Majuro Atoll. In this study, 563 vascular plants have been recorded on Majuro. INTRODUCTION The accompanying report presents a complete flora of Majuro Atoll, which has never been done before. It includes a listing of all species, notation as to origin (i.e. indigenous, aboriginal introduction, recent introduction), as well as the original range of each. The major synonyms are also listed. For almost all, English common names are presented. Marshallese names are given, where these were found, and spelled according to the current spelling system, aside from limitations in diacritic markings. A brief notation of location is given for many of the species. The entire list of 563 plants is provided to give the people a means of gaining a better understanding of the nature of the plants of Majuro Atoll. -

Identification and Characterization of Macrophomina Phaseolina Causing Leaf Blight on White Spider Lilies (Crinum Asiaticum and Hymenocallis Littoralis) in Malaysia
MYCOBIOLOGY 2019, VOL. 47, NO. 4, 408–414 https://doi.org/10.1080/12298093.2019.1682448 RESEARCH ARTICLE Identification and Characterization of Macrophomina phaseolina Causing Leaf Blight on White Spider Lilies (Crinum asiaticum and Hymenocallis littoralis) in Malaysia Abd Rahim Huda-Shakirah , Yee Jia Kee , Abu Bakar Mohd Hafifi , Nurul Nadiah Mohamad Azni, Latiffah Zakaria and Masratul Hawa Mohd School of Biological Sciences, Universiti Sains Malaysia, Penang, Malaysia ABSTRACT ARTICLE HISTORY Crinum asiaticum and Hymenocallis littoralis, commonly known as spider lilies are bulbous Received 11 March 2019 perennial and herbaceous plants that widely planted in Malaysia as ornamental. During Revised 3 August 2019 2015–2016, symptom of leaf blight was noticed on the hosts from several locations in Accepted 15 October 2019 Penang. The symptom appeared as irregular brown to reddish lesions surrounded by yellow KEYWORDS halos. As the disease progressed, the infected leaves became blighted, dried, and fell off Macrophomina phaseolina; with the presence of black microsclerotia and pycnidia on the lesions parts. The present leaf blight; Crinum study was conducted to investigate the causal pathogen of leaf blight on C. asiaticum and asiaticum; H. littoralis. Based on morphological characteristics and DNA sequences of internal tran- Hymenocallis littoralis scribed spacer (ITS) region and translation elongation factor 1-alpha (TEF1-a) gene, the causal pathogen was identified as Macrophomina phaseolina. Phylogenetic analysis of com- bined dataset of ITS and TEF1-a grouped the isolates studied with other isolates of M. pha- seolina from GenBank. The grouping of the isolates was supported by 96% bootstrap value. Pathogenicity test proved the role of the fungus in causing leaf blight on both hosts. -

Networks in a Large-Scale Phylogenetic Analysis: Reconstructing Evolutionary History of Asparagales (Lilianae) Based on Four Plastid Genes
Networks in a Large-Scale Phylogenetic Analysis: Reconstructing Evolutionary History of Asparagales (Lilianae) Based on Four Plastid Genes Shichao Chen1., Dong-Kap Kim2., Mark W. Chase3, Joo-Hwan Kim4* 1 College of Life Science and Technology, Tongji University, Shanghai, China, 2 Division of Forest Resource Conservation, Korea National Arboretum, Pocheon, Gyeonggi- do, Korea, 3 Jodrell Laboratory, Royal Botanic Gardens, Kew, Richmond, United Kingdom, 4 Department of Life Science, Gachon University, Seongnam, Gyeonggi-do, Korea Abstract Phylogenetic analysis aims to produce a bifurcating tree, which disregards conflicting signals and displays only those that are present in a large proportion of the data. However, any character (or tree) conflict in a dataset allows the exploration of support for various evolutionary hypotheses. Although data-display network approaches exist, biologists cannot easily and routinely use them to compute rooted phylogenetic networks on real datasets containing hundreds of taxa. Here, we constructed an original neighbour-net for a large dataset of Asparagales to highlight the aspects of the resulting network that will be important for interpreting phylogeny. The analyses were largely conducted with new data collected for the same loci as in previous studies, but from different species accessions and greater sampling in many cases than in published analyses. The network tree summarised the majority data pattern in the characters of plastid sequences before tree building, which largely confirmed the currently recognised phylogenetic relationships. Most conflicting signals are at the base of each group along the Asparagales backbone, which helps us to establish the expectancy and advance our understanding of some difficult taxa relationships and their phylogeny. -

Cairns Regional Council Specific Requirments
LANDSCAPING LOCAL GOVERNMENT SPECIFIC REQUIREMENTS INTRODUCTION This section contains variations and additions to FNQROC Regional Development Manual that apply specifically within the Cairns Regional Council Local Government Area. CONTENTS The following sections have varied or additional clauses: CLAUSE PAGE CONSTRUCTION PROCEDURES - CP1 ............................................................................. 3 CP1.17 APPLICATION FOR COUNCIL TO COMPLETE PRIVATE WORKS............................................. 3 APPENDIX P – 3 4. DRAFTING REQUIREMENTS (“AS CONSTRUCTED”) ................................................................................. 3 12. WATER RETICULATION .............................................................................................................................. 4 13. SEWERAGE RETICULATION ....................................................................................................................... 5 15.STORMWATER DRAINAGE SYSTEM .......................................................................................................... 5 DESIGN GUIDELINE – D1 ROAD GEOMETRY ................................................................... 6 D1.22 SIGNS AND ROAD MARKINGS ..................................................................................................... 6 DESIGN GUIDELINE – D2 SITE REGRADING .................................................................... 6 D2.05 CLEARING ..................................................................................................................................... -

National Wetland Plant List: 2016 Wetland Ratings
Lichvar, R.W., D.L. Banks, W.N. Kirchner, and N.C. Melvin. 2016. The National Wetland Plant List: 2016 wetland ratings. Phytoneuron 2016-30: 1–17. Published 28 April 2016. ISSN 2153 733X THE NATIONAL WETLAND PLANT LIST: 2016 WETLAND RATINGS ROBERT W. LICHVAR U.S. Army Engineer Research and Development Center Cold Regions Research and Engineering Laboratory 72 Lyme Road Hanover, New Hampshire 03755-1290 DARIN L. BANKS U.S. Environmental Protection Agency, Region 7 Watershed Support, Wetland and Stream Protection Section 11201 Renner Boulevard Lenexa, Kansas 66219 WILLIAM N. KIRCHNER U.S. Fish and Wildlife Service, Region 1 911 NE 11 th Avenue Portland, Oregon 97232 NORMAN C. MELVIN USDA Natural Resources Conservation Service Central National Technology Support Center 501 W. Felix Street, Bldg. 23 Fort Worth, Texas 76115-3404 ABSTRACT The U.S. Army Corps of Engineers (Corps) administers the National Wetland Plant List (NWPL) for the United States (U.S.) and its territories. Responsibility for the NWPL was transferred to the Corps from the U.S. Fish and Wildlife Service (FWS) in 2006. From 2006 to 2012 the Corps led an interagency effort to update the list in conjunction with the U.S. Environmental Protection Agency (EPA), the FWS, and the USDA Natural Resources Conservation Service (NRCS), culminating in the publication of the 2012 NWPL. In 2013 and 2014 geographic ranges and nomenclature were updated. This paper presents the fourth update of the list under Corps administration. During the current update, the indicator status of 1689 species was reviewed. A total of 306 ratings of 186 species were changed during the update. -

Atoll Research Bulletin No. 392 the Flora of Nauru Rr
ATOLL RESEARCH BULLETIN NO. 392 THE FLORA OF NAURU RR THAMAN, F.R FOSBERG, EL MANNER AND D.C. HASSALL ISSUED BY NATIONAL MUSEUM OF NATURAL J!WTORY SMllTJ!WNIAN INSTlTUTION WASHINGTON, D.C, USA FEBRUARY 1994 DEDICATION We dedicate this Flora of Nauru to Joseph Detsimea Audoa, his family and the people of the Republic of Nauru who have had their precious island and its flora destroyed and degraded as a result of wars and exploitation beyond their control. ACKNOWLEDGEMENTS The authors would like to acknowledge, in particular, the late Honorable Joseph Detsimea Audoa, the Minister of Health and Education at the time of the commencement of the study and later Minister of Justice in the Government of Nauru, who, because of his vision and commitment to the culture and environment of Nauru, initiated and provided the financial support for the study of the flora of Nauru. He was particularly concerned that the plants of Nauru and their cultural uses be recorded before such knowledge was lost. We also acknowledge Mr. Lisle Newby, the then Director of Education, who, along with Joe Audoa, were the main supporters of the project, and who provided valuable logistical support throughout. Special thanks are also given to our main local informants and assistants, the Reverend James Aingimea and the late Henry Michael Heine; and to Daphne Fotu, Jacob Gabwinare, Katarina Satto, Kenia Raidinen, Reynold Capelle, Eda Adam and Montiba Star, our main informants in relation to the cultural uses and Nauruan names of plants. Our thanks also go to the Honorable Lawrence Stephen, Minister of Education during part of the project; Obera Menke, Robert Kaierua, Leo Keke, Delilah Capelle, Eddie Borak, John Healy, Gary Bailey, Dennis and Ria Berdinner, Julie Olsson, Dennis Ketner, Sio Fotu, Pine Harrison, John Brechtefeld, Rene Harris, Porthos Bop, Jacob Aroi, Leon Thompson, Benjamin Morgan, Iosefa Elisala and Teaora Tabanou, all of whom contributed in some way to the success of the study. -

In Vitro Propagation of Haemanthus Pumilio and H. Albiflos (Amaryllidaceae) and the Population Genetics of H
In Vitro Propagation of Haemanthus pumilio and H. albiflos (Amaryllidaceae) and the population genetics of H. pumilio By Panashe Kundai Madzivire Thesis presented in partial fulfilment of the requirements for the degree of Master of Science in the Faculty of Science at Stellenbosch University Supervisor: Paul N Hills Co-supervisor: Léanne L. Dreyer March 2021 i Stellenbosch University https://scholar.sun.ac.za DECLARATION By submitting this thesis electronically, I declare that the entirety of the work contained therein is my own, original work, that I am the sole author thereof (save to the extent explicitly otherwise stated), that reproduction and publication thereof by Stellenbosch University will not infringe any third party rights and that I have not previously in its entirety or in part submitted it for obtaining any qualification Copyright © 2021 Stellenbosch University All rights reserved ii Stellenbosch University https://scholar.sun.ac.za ENGLISH - ABSTRACT Haemanthus albiflos and H. pumilio are members of the Amaryllidaceae. H. albiflos is a widespread evergreen plant, while H. pumilio is an endangered narrow endemic species with only two known populations remaining. These populations, remnants of the one recently transferred from Wellington to the Stellenbosch University Botanical gardens and another in the Duthie Reserve in Stellenbosch, present with vastly different morphologies. It is therefore vital to understand the phylogenetics and population genetics of H. pumilio as well as to design a method of in vitro propagation to increase the numbers of these plants. This study analysed the phylogenetics of H. pumilio using non-coding nuclear (Internal transcribed spacer), plastid (trnL-trnF intergenic spacer) and mitochondrial (nadi1477) gene regions and the population genetics using inter-simple sequence repeats (ISSR) and start codon targeted (SCoT) polymorphisms. -

Research on the Alkaloids of Amaryllidaceae Plants: Genera Lycoris and Hippeastrum
Research on the Alkaloids of Amaryllidaceae Plants: Genera Lycoris and Hippeastrum Ying Guo ADVERTIMENT . La consulta d’aquesta tesi queda condicionada a l’acceptació de les següents condicions d'ús: La difusió d’aquesta tesi per mitjà del servei TDX ( www.tdx.cat ) i a través del Dipòsit Digital de la UB ( diposit.ub.edu ) ha estat autoritzada pels titulars dels drets de propietat intel·lectual únicament per a usos privats emmarcats en a ctivitats d’investigació i docència. No s’autoritza la seva reproducció amb finalitats de lucre ni la seva difusió i posada a disposici ó des d’un lloc aliè al servei TDX ni al Dipòsit Digital de la UB . No s’autoritza la presentació del seu contingut en una finestra o marc aliè a TDX o al Dipòsit Digital de la UB (framing). Aquesta reserva de drets afecta tant al resum de presentació de la tesi com als seus continguts. En la utilització o cita de parts de la tesi és obligat indicar el nom de la persona autor a. ADVERTENCIA . La consulta de esta tesis queda condicionada a la aceptación de las siguientes condiciones de uso: La difusión de esta tesis por medio del servicio TDR ( www.tdx.cat ) y a través del Repositorio Digital de la UB ( diposit.ub.edu ) ha sido autorizada por los titulares de los derechos de propiedad intelectual únicamente para usos privados enmarcados en actividades de investigación y docencia. No se autoriza su reproducción con finalidades de lucro ni su difusión y puesta a disposición desde u n sitio ajeno al servicio TDR o al Repositorio Digital de la UB .