Plum Pox Virus
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Pru Nus Contains Many Species and Cultivars, Pru Nus Including Both Fruits and Woody Ornamentals
;J. N l\J d.000 A~ :J-6 '. AGRICULTURAL EXTENSION SERVICE UNIVERSITY OF MINNESOTA • The genus Pru nus contains many species and cultivars, Pru nus including both fruits and woody ornamentals. The arboretum's Prunus maacki (Amur Cherry). This small tree has bright, emphasis is on the ornamental plants. brownish-yellow bark that flakes off in papery strips. It is par Prunus americana (American Plum). This small tree furnishes ticularly attractive in winter when the stems contrast with the fruits prized for making preserves and is also an ornamental. snow. The flowers and fruits are produced in drooping racemes In early May, the trees are covered with a "snowball" bloom similar to those of our native chokecherry. This plant is ex of white flowers. If these blooms escape the spring frosts, tremely hardy and well worth growing. there will be a crop of colorful fruits in the fall. The trees Prunus maritima (Beach Plum). This species is native to the sucker freely, and unless controlled, a thicket results. The A coastal plains from Maine to Virginia. It's a sprawling shrub merican Plum is excellent for conservation purposes, and the reaching a height of about 6 feet. It blooms early with small thickets are favorite refuges for birds and wildlife. white flowers. Our plants have shown varying degrees of die Prunus amygdalus (Almond). Several cultivars of almonds back and have been removed for this reason. including 'Halls' and 'Princess'-have been tested. Although Prunus 'Minnesota Purple.' This cultivar was named by the the plants survived and even flowered, each winter's dieback University of Minnesota in 1920. -

Cambridge University Press 978-1-107-11607-8 — a Natural History of Ladybird Beetles M. E. N. Majerus , Executive Editor H. E. Roy , P
Cambridge University Press 978-1-107-11607-8 — A Natural History of Ladybird Beetles M. E. N. Majerus , Executive Editor H. E. Roy , P. M. J. Brown Index More Information Index 2-isopropyl-3-methoxy-pyrazine, 238 281, 283, 285, 287–9, 291–5, 297–8, 2-phenylethylamine, 237 301–3, 311, 314, 316, 319, 325, 327, 329, 335 abdomen, 17, 20, 22, 24, 28–9, 32, 38, 42, 110, Adalia 4-spilota,80 114, 125, 128, 172, 186, 189, 209–10, Adalia conglomerata, 255 218 adaline, 108, 237, 241 Acacia, 197, 199 adalinine, 237 acaricides, 316 adelgids, 29, 49, 62, 65, 86, 91, 176, 199, 308, Acaridae, 217 310, 322 Acarina, 205, 217 Adonia, 44, 71 Acer pseudoplatanus, 50, 68, 121 aggregations, 163, 165, 168, 170, 178, 184, Acraea, 228, 297, 302 221, 312, 324 Acraea encedana, 302 Aiolocaria, 78, 93, 133, 276 Acraea encedon, 297, 302 Aiolocaria hexaspilota,78 Acyrthosiphon nipponicum, 101 Aiolocaria mirabilis, 133, 276 Acyrthosiphon pisum, 75, 77, 90, 92, 97–101, albino, 273 116, 239 Alces alces,94 Adalia, 5–6, 10, 22, 34, 44, 64, 70, 78, 80, 86, Aleyrodidae, 91, 310 123, 125, 128, 130, 132, 140, 143, 147, alfalfa, 119, 308, 316, 319, 325 159–60, 166–7, 171, 180–1, 218, 222, alimentary canal, 29, 35, 221 234, 237, 239, 241, 255, 259–60, 262, alkaloids, x, 99–100, 195–7, 202, 236–9, 241–2, 269, 279, 281, 284, 286, 298, 311, 325, 245–6 327, 335 Allantonematidae, 220 Adalia 10-punctata, 22, 70, 80, 86, 98–100, anal cremaster, 38, 40 104, 108, 116, 132, 146–7, 149, Anatis, 4, 17, 23, 41, 44, 66, 76, 89, 102, 131, 154, 156, 160, 174, 181–3, 188, 148, 165, 186, 191, 193, -

EN ANNEX Annex I Definitions As Referred to in Article 2(1)
Ref. Ares(2019)5150577 - 08/08/2019 EN ANNEX Annex I Definitions as referred to in Article 2(1) For the purposes of this Regulation, the terms listed in Part A, when used in the Annexes to this Regulation, have the same meaning as defined in the respective Directives listed in the second column of Part B. Part A List of terms - Pre-basic seed; - Basic seed; - Certified seed; - Standard seed; - Vine; - Initial propagating material; - Basic propagating material; - Certified material; - Ornamental plants; - Forest reproductive material; - Commercial seed; - Vegetable propagating and planting material; - Candidate pre-basic mother plant; - Pre-basic material; - Pre-basic mother plant; - Basic mother plant; - Basic material; - Certified mother plant; - Conformitas Agraria Communitatis (CAC) material; - Cereal seed; - Vegetable seed; - Oil and fibre plants seed 1 Part B List of Directives and Annexes 1. ANNEXES TO THIS REGULATION 2. DIRECTIVES ANNEX IV, Part A Council Directive 66/401/EEC (RNQPs concerning fodder plant seed) ANNEX V, Part A (Measures concerning fodder plant seed) ANNEX IV, Part B Council Directive 66/402/EEC (RNQPs concerning cereal seed) ANNEX V, Part B (Measures concerning cereal seed) ANNEX IV, Part C Council Directive 68/193/EEC (RNQPs concerning vine, other than vine grown in production places or sites, the presence of which is subject to visual inspection only) ANNEX IV, Part D Council Directive 98/56/EC (RNQPs, the presence of which is subject to visual inspection only, concerning ornamental plants, other than ornamental -

Plums on the Prairies by Rick Sawatzky
Plums on the Prairies by Rick Sawatzky Information from Literature Much has been published about pollination, pollinators, pollinizers, fertilization and fruit set in text books and periodicals. The definitions are not difficult. Pollination is the movement of pollen among compatible flowering plants (cross-pollination) or from anthers to stigmas on the same plant or different plants of the same clone (self-pollination). Many plants will self-pollinate but set very few fruit; some authors consider them self- pollinating but they are definitely not self-fruitful. Self-fruitful plants (and clones) set a crop of fruit after self-pollination; some of these plants bear fruit with no seeds (parthenocarpy); others develop seeds with embryos that are genetically identical to the parent plant (apomixis); and others produce haploid seeds that develop from an unfertilized egg cell. (When haploid seeds germinate they are very weak seedlings with only half the chromosomes of normal seedlings.) Regarding temperate zone tree fruits, self-pollination and fruit set does not mean self-fertility and the development of normal seeds. Many temperate zone small fruit species (e.g. strawberries and raspberries) are self-fertile and develop maximum yields of fruit with normal seeds as the result of self-pollination by insects. Pollinators, usually insects, are vectors of pollen movement. Pollinizers are plants which provide the appropriate pollen for other plants. Fertilization is the process in which gametes from the pollen unite with egg cells in the ovary of the flower. Normal seeds are usually the result of this process. Also, the principles are easily understood. Poor fertilization in plums and other Prunus species results in a poor fruit set. -

Checklist of Illinois Native Trees
Technical Forestry Bulletin · NRES-102 Checklist of Illinois Native Trees Jay C. Hayek, Extension Forestry Specialist Department of Natural Resources & Environmental Sciences Updated May 2019 This Technical Forestry Bulletin serves as a checklist of Tree species prevalence (Table 2), or commonness, and Illinois native trees, both angiosperms (hardwoods) and gym- county distribution generally follows Iverson et al. (1989) and nosperms (conifers). Nearly every species listed in the fol- Mohlenbrock (2002). Additional sources of data with respect lowing tables† attains tree-sized stature, which is generally to species prevalence and county distribution include Mohlen- defined as having a(i) single stem with a trunk diameter brock and Ladd (1978), INHS (2011), and USDA’s The Plant Da- greater than or equal to 3 inches, measured at 4.5 feet above tabase (2012). ground level, (ii) well-defined crown of foliage, and(iii) total vertical height greater than or equal to 13 feet (Little 1979). Table 2. Species prevalence (Source: Iverson et al. 1989). Based on currently accepted nomenclature and excluding most minor varieties and all nothospecies, or hybrids, there Common — widely distributed with high abundance. are approximately 184± known native trees and tree-sized Occasional — common in localized patches. shrubs found in Illinois (Table 1). Uncommon — localized distribution or sparse. Rare — rarely found and sparse. Nomenclature used throughout this bulletin follows the Integrated Taxonomic Information System —the ITIS data- Basic highlights of this tree checklist include the listing of 29 base utilizes real-time access to the most current and accept- native hawthorns (Crataegus), 21 native oaks (Quercus), 11 ed taxonomy based on scientific consensus. -

Italy: First Steps to Be Taken
The National Crop Wild Relative Strategy for Italy: First Steps To Be Taken PGR Secure The National Crop Wild Relative Strategy for Italy: First Steps To Be Taken * Panella L. 1, Landucci S. 12, Torricelli R. 1, Gigante D. 13, Donnini D. 1, Venanzoni R.13 and V. Negri1 1 Department of Agricultural, Nutritional and Environmental Sciences, University of Perugia, Borgo XX Giugno 74, 06121 Perugia, Italy 2 Department of Botany and Zoology, Masaryk University, Kotlárská 2, Brno 61137 (present address) 3 Department of Chemistry, Biology and Biotechnology, University of Perugia, via Elce di Sotto 8, 06123 Perugia, Italy (present address) * Largely based on Landucci et al. (2014). A prioritized inventory of crop wild relatives and harvested plants of Italy. Crop Science. doi: 10.2135/cropsci2013.05.0355. Index 1. INTRODUCTION ................................................................................................................................................. 4 1.1 DEFINITION OF A CROP WILD RELATIVE ....................................................................................................... 4 1.2 CROP WILD RELATIVE CONSERVATION AND INTERNATIONAL TREATIES .............................................. 4 1.3 ITALIAN IMPLEMENTATION OF THE PLANT CONSERVATION STRATEGIES .............................................. 5 1.4 GENETIC RESOURCES OF THE MEDITERRANEAN BASIN AND OF ITALY .................................................. 6 1.5 ITALIAN PROTECTED AREAS AND SPECIES ..................................................................................................... -

The Buffer Handbook Plant List
THE BUFFER HANDBOOK PLANT LIST Originally Developed by: Cynthia Kuhns, Lake & Watershed Resource Management Associates With funding provided by U.S. Environmental Protection Agency and Maine Department of Environmental Protection,1998. Revised 2001 and 2009. Publication #DEPLW0094-B2009 TABLE OF CONTENTS Page Acknowledgements 1 Introductory Information Selection of Plants for This List 1 Plant List Organization & Information 3 Terms & Abbreviations 4 Plant Hardiness Zone Map 5 General Tree & Shrub Planting Guidelines 5 Tips for Planting Perennials 7 Invasive Plants to Avoid 7 Plant Lists TREES 8 (30 to 100 ft.) SHRUBS 14 Small Trees/Large Shrubs 15 (12 to 30 ft.) Medium Shrubs 19 (6 to 12 ft.) Small Shrubs 24 (Less than 6 ft.) GROUNDLAYERS 29 Perennial Herbs & Flowers 30 Ferns 45 Grasses 45 Vines 45 References 49 ACKNOWLEDGEMENTS Original Publication: This plant list was published with the help of Clean Water Act, Section 319 funds, under a grant awarded to the Androscoggin Valley Soil and Water Conservation District and with help from the Maine Department of Environmental Protection and the U.S. Environmental Protection Agency. Graphics and ‘clip-art’ used in this document came from the University of Wisconsin-Extension and from Microsoft Office 97(Small Business Edition) and ClickArt 97 (Broderbund Software, Inc). This publication was originally developed by Cynthia Kuhns of Lake & Watershed Resource Management Associates. Substantial assistance was received from Phoebe Hardesty of the Androscoggin Valley Soil and Water Conservation District. Valuable review and advice was given by Karen Hahnel and Kathy Hoppe of the Maine Department of Environmental Protection. Elizabeth T. Muir provided free and cheerful editing and botanical advice. -
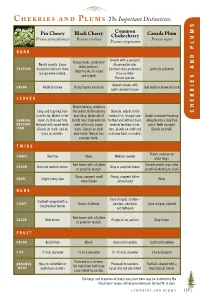
C P the Important Distinctions
C P The Important Distinctions S Common M Pin Cherry Black Cherry Chokecherry Canada Plum Prunus pennsylvanica Prunus serotina Prunus nigra U Prunus virginiana L P BARK D Smooth with a pungent, Young trunks: prominent N Nearly smooth. Large disagreeable odor. white lenticals. TEXTURE horizontal lenticels show Lenticels less prominent Lenticels yellowish A Older trunks: fissured orange when rubbed. than on other and ridged. Prunus species. S E Grayish-brown, with I COLOR Reddish-brown Young trunks are black Dull reddish-brown to black light-colored fissures R LEAVES R E Elliptic/oblong, widest in H Long and tapering from the center, thick leathery Obovate, widest in the C base to tip. Widest in the and shiny. Underside of terminal 1⁄3, sharply saw- Ovate or obovate tapering GENERAL lower 1⁄3; thin and firm midrib near stalk end cov- toothed and without hairs, abruptly into a long thin DESCRIP- textured with round teeth. ered with rusty, brown medium leathery in tex- point. Teeth rounded. TION Glands on stalk, and no hairs. Glands on stalk ture, glands on stalk and Glands on stalk. hairs on midribs. near blade. Margin has no brown hairs on midrib. rounded teeth. TWIGS Thorns common on SHAPE Very fine Waxy Medium slender older twigs Red-brown with a lighter Current growth gray, older COLOR Red and reddish-brown Gray or purplish-brown or greenish margin growth darkening to black Sharp, pungent smell Strong, pungent bitter- ODOR Slight cherry odor None when broken almond odor BUDS Cone shaped, slender – Football-shaped with a SHAPE Ovate, -

Coccinellidae)
ECOLOGY AND BEHAVIOUR OF THE LADYBIRD BEETLES (COCCINELLIDAE) Edited by I. Hodek, H.E van Emden and A. Honek ©WILEY-BLACKWELL A John Wiley & Sons, Ltd., Publication CONTENTS Detailed contents, ix 8. NATURAL ENEMIES OF LADYBIRD BEETLES, 375 Contributors, xvii Piotr Ccryngier. Helen E. Roy and Remy L. Poland Preface, xviii 9. COCCINELLIDS AND [ntroduction, xix SEMIOCHEMICALS, 444 ]an Pettcrsson Taxonomic glossary, xx 10. QUANTIFYING THE IMPACT OF 1. PHYLOGENY AND CLASSIFICATION, 1 COCCINELLIDS ON THEIR PREY, 465 Oldrich Nedved and Ivo Kovdf /. P. Mid'laud and James D. Harwood 2. GENETIC STUDIES, 13 11. COCCINELLIDS IN BIOLOGICAL John J. Sloggett and Alois Honek CONTROL, 488 /. P. Midland 3. LIFE HISTORY AND DEVELOPMENT, 54 12. RECENT PROGRESS AND POSSIBLE Oldrkli Nedved and Alois Honek FUTURE TRENDS IN THE STUDY OF COCCINELLIDAE, 520 4. DISTRIBUTION AND HABITATS, 110 Helmut /; van Emden and Ivo Hodek Alois Honek Appendix: List of Genera in Tribes and Subfamilies, 526 5. FOOD RELATIONSHIPS, 141 Ivo Hodek and Edward W. Evans Oldrich Nedved and Ivo Kovdf Subject index. 532 6. DIAPAUSE/DORMANCY, 275 Ivo Hodek Colour plate pages fall between pp. 250 and pp. 251 7. INTRAGUILD INTERACTIONS, 343 Eric Lucas VII DETAILED CONTENTS Contributors, xvii 1.4.9 Coccidulinae. 8 1.4.10 Scymninae. 9 Preface, xviii 1.5 Future Perspectives, 10 References. 10 Introduction, xix Taxonomic glossary, xx 2. GENETIC STUDIES, 13 John J. Sloggett and Alois Honek 1. PHYLOGENY AND CLASSIFICATION, 1 2.1 Introduction, 14 Oldrich Nedved and Ivo Kovdf 2.2 Genome Size. 14 1.1 Position of the Family. 2 2.3 Chromosomes and Cytology. -

Phragmites Australis
Journal of Ecology 2017, 105, 1123–1162 doi: 10.1111/1365-2745.12797 BIOLOGICAL FLORA OF THE BRITISH ISLES* No. 283 List Vasc. PI. Br. Isles (1992) no. 153, 64,1 Biological Flora of the British Isles: Phragmites australis Jasmin G. Packer†,1,2,3, Laura A. Meyerson4, Hana Skalov a5, Petr Pysek 5,6,7 and Christoph Kueffer3,7 1Environment Institute, The University of Adelaide, Adelaide, SA 5005, Australia; 2School of Biological Sciences, The University of Adelaide, Adelaide, SA 5005, Australia; 3Institute of Integrative Biology, Department of Environmental Systems Science, Swiss Federal Institute of Technology (ETH) Zurich, CH-8092, Zurich,€ Switzerland; 4University of Rhode Island, Natural Resources Science, Kingston, RI 02881, USA; 5Institute of Botany, Department of Invasion Ecology, The Czech Academy of Sciences, CZ-25243, Pruhonice, Czech Republic; 6Department of Ecology, Faculty of Science, Charles University, CZ-12844, Prague 2, Czech Republic; and 7Centre for Invasion Biology, Department of Botany and Zoology, Stellenbosch University, Matieland 7602, South Africa Summary 1. This account presents comprehensive information on the biology of Phragmites australis (Cav.) Trin. ex Steud. (P. communis Trin.; common reed) that is relevant to understanding its ecological char- acteristics and behaviour. The main topics are presented within the standard framework of the Biologi- cal Flora of the British Isles: distribution, habitat, communities, responses to biotic factors and to the abiotic environment, plant structure and physiology, phenology, floral and seed characters, herbivores and diseases, as well as history including invasive spread in other regions, and conservation. 2. Phragmites australis is a cosmopolitan species native to the British flora and widespread in lowland habitats throughout, from the Shetland archipelago to southern England. -

SPRING FLOWERING TREES for COLORADO LANDSCAPE Revised 12/9/2019
SPRING FLOWERING TREES FOR COLORADO LANDSCAPE Revised 12/9/2019 Craig R. Miller Parks & Open Space Manager www.cpnmd.org Types of Flowering Trees: • Fruit Trees - Apples, Apricots, Cherries, Peaches, Plums • Crabapples • Hawthorns • Ornamental Pears/Plums • Horsechestnuts • Other - Goldenrain Tree, Tree Lilac, Redbud, Serviceberry, Smoke Tree Fruit Trees Apple (Malus spp.) • Pink buds open to white flowers. • Many varieties to choose from including Gala, Red Delicious and Honeycrisp™. • Apple trees need another variety to produce fruit. • 15’ to 20’ height, 10’ to 18’ spread; max. elevation 8,000 ft. Chinese Apricot (Prunus armeniaca ‘Chinese’) • An early-bearing variety that is ideal for difficult climates prone to late spring frosts. • Mildly fragrant, early white blooms open from pink buds. • Fruit is medium in size and sweet. Self-fertile. • 15’ height, 10’ spread; max. elevation 6,000 ft. Montmorency Cherry (Prunus cerasus ‘Montmorency’) • The finest sour or pie cherry. A heavy annual bearer, the average yield for a mature tree is 36–44 pints; self-pollinating. • A vigorous tree with upright, spreading branches and a rounded crown, it bears fruit at a young age. • Late bloom means a more dependable production of fruit; ripens in June or early July. • 15’ height and 10’ spread; max. elevation 7,500 ft. Redhaven Peach (Prunus persica ‘Redhaven’) • Developed in Michigan, this variety has become the gold standard for peaches, replacing Elberta as the most versatile and widely grown. • Regular high yields make this one of the best home garden peaches. • Vulnerable to late spring damage due to early bloom time, ripens mid-August. • 15’ height and 12’ spread; max. -

University of California, Berkeley, Department of Environmental Science, Policy, and Management
Jeffrey D. Lozier (PI: N.J. Mills, Collaborator: G.K. Roderick) University of California, Berkeley, Department of Environmental Science, Policy, and Management TheThe GeneticsGenetics andand BiologicalBiological ControlControl ofof HyalopterusHyalopterus pruni,pruni, anan InvasiveInvasive AphidAphid PestPest inin CaliforniaCalifornia 1. Abstract 3. Research Approaches • The mealy plum aphid, Hyalopterus pruni, is an invasive pest on prune in California. The genus Hyalopterus originates from the Mediterranean, B. Virulence Experiments and is currently believed to be comprised of two Hap1 indistinguishable species that feed on multiple • Virulence is a parasitoid’s ability to overcome its host’s defenses and successfully reproduce. hosts in the genus Prunus. A. Molecular Methods Hap2a • Biological control using Aphidius transcaspicus, 1) Examine the population structure of H. pruni • I will expose multiple geographic populations of and A. transcaspicus in the Mediterranean and A. transcaspicus to Californian H. pruni in cages a parasitoid from H. pruni’s ancestral home, is a Hap2b potential way to mediate the effect of this pest. clarify issues surrounding their taxonomy. to examine reproductive success and determine • I will use DNA sequences for the mitochondrial the relative virulence of each parasitoid strain. • Using molecular genetics and ecological Hap3a (mtDNA) COI gene to look for deeper level experiments I will characterize the population • In correlation with genetic data, this will provide differences within the two taxa, such as biotypes, structure of these two species and their valuable information on the structure of mealy host races, or even cryptic species. interactions to aid the design of an effective aphid-parasitoid interactions and help identify the Hap3b biocontrol program for mealy aphid in California.