Red Meat and Processed Meat Volume 114
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Behind the Charcuterie Board
Behind the Charcuterie Board SID WAINER & SON We present to you an exquisite collection SALAMI of premium charcuterie specialties that are sure to adorn your cheese and charcuterie JANSAL VALLEY boards and drive deep savory flavor into WILD BOAR SALAMI culinary creations of your choice. 100% Texas wild boar meat, mixed with fresh herbs and wine for an authentic If you are in search of an item that you do Mediterranean flavor. not see within the following presentation, #346360 6/5.5 oz please do not hesitate to contact us. It will JANSAL VALLEY be our pleasure to find it for you. CURED DUCK SALAMI 100% duck meat with red wine and black peppercorns, aged for 2 months. Bon appétit, #346370 6/6 oz Sid Wainer & Son CREMINELLI BAROLO SALAMI Uncured Italian salami, made with Barolo red wine and crafted without the use of artificial ingredients. #349120 9/5.5 oz CREMINELLI FINOCCHIONA SALAMI Coarsely ground salami mixed with organic fennel and spices, crafted without artificial ingredients. #349140 3/2.75 lb CREMINELLI SOPRESSATA SALAMI Uncured Italian salami, with no artificial ingredients, using a northern Italian recipe with garlic and wine. #349150 9/5.5 oz CREMINELLI SOPRESSATA TARTUFO SALAMI Uncured Italian salami, infused with black JANSAL VALLEY summer truffles and crafted without the use SWEET BABY SOPRESSATA of artificial ingredients. Crafted from coarsely ground premium pork #349180 9/5.5 oz and made without the use of additives or fillers. Each piece is dry aged for a minimum of 30 days DOMAINE DE PROVENCE to develop a firm texture and well-balanced SAUCISSON SEC SALAMI savory taste. -

Cookies P.9 Summer Fancy Food Show, Part I P.13 World Cheese Book
Editorial Views p.3 The Sideboard: Cookies p.9 Report From the Trenches: Summer Fancy Food Show, Part I p.13 From the Deli Bookshelf World Cheese Book p.18 Rules & Regulations p.21 Passport to Fine Charcuterie p.5 Volume 5, Issue 5 August 2015 FabriqueDélices French Artistry on the Shores of SF Bay www.gourmetbusiness.com Fabrique Délices French Artistry on the Shores of SF Bay CHARCUTERIE Well-made charcuterie, whether it’s for the ideal picnic described at left, a By James Mellgren little something to accompany a glass of wine at the end of the day, or as an array of charcuterie. In fact, I recently LQJUHGLHQWLQ\RXUIDYRULWHGLVKHVLV KDGWKHJUHDWSOHDVXUHRIYLVLWLQJRQHRI RQHRIWKHJUHDWMR\VRIPRGHUQOLYLQJ the best-known producers of authentic, A charcuterie in Aurillac or Vic-sur-Cère or some other small but Many cultures throughout the world artisan charcuterie in the United States, make some form of cured meats and locally important town will possibly provide a pâté the like of which DQGOXFN\IRUPHWKH\DUHDVKRUWGULYH meat pastes, but it is to the traditions of away from my home, situated just south you never tasted before, or a locally cured ham, a few slices of which France that we turn here. The word we of Oakland, near the shores of the San use in our business most to describe this you will buy and carry away with a salad, a kilo of peaches, a bottle Francisco Bay in Hayward, Calif. category of gourmet foods, charcuterie, of Monbazillac and a baton of bread, and somewhere on a hillside comes from the French term cuiseur de Fabrique Délices began in 1985, set up chair, or “cooker of meats,” but today RULJLQDOO\DVDVXEVLGLDU\RI6$3$5 amid the mile upon mile of golden broom or close to a splashing it also refers to a shop (or department the leading pâté producer in France waterfall you will have, just for once, the ideal picnic. -

Cheese & Charcuterie
WE PROUDLY SERVE CALVISIUS CAVIAR • AVAILABLE IN 28G, 50G, AND 125G PORTIONS CAVIAR SERVED WITH FRESH ACCOUTREMENTS, CREME FRAICHE AND POTATO BLINI OSCIETRA SEVRUGA OSCIETRA ROYAL IMPERIAL GOLD Exclusive to Wally’s small black to light gray large golden amber large golden beads with beads, soft eggs reminis- beads with rich, creamy, umami flavors of pine nuts, cent of anchovies bright notes pecans and seafoam brine 250 • 415 • 975 190 • 305 • 700 175 • 280 • 640 SIBERIAN ROYAL TRADITION PRESTIGE small light gray beads large grey-black caviar with with succulent, savory elegant buttery, creamy flavors of cured meat flavors and dried fruit 115 • 170 • 370 130 • 190 • 415 CHEESE & CHARCUTERIE PLATES 3 FOR 25 | 5 FOR 40 • CHOOSE FROM BELOW CHEESE CHARCUTERIE CHALLERHOCKER “5J” JAMON IBERICO DE BELLOTA (ADD 20) Switzerland / raw cow - brothy, nutty, hint of cacao pork - buttery, nutty, rich, decadent HOOKS 10YR CHEDDAR (ADD 10) GIORGIO’S FINOCCHIONA Wisconsin / cow - intense, rich, tangy pork - fennel seed, anise SHROPSHIRE BLUE PIO TOSINI PROSCIUTTO DI PARMA England / cow - smooth, fruity, tangy pork - nutty, supple, fruity L’AMUSE GOUDA CHARLITO’S TRUFFLE SALAMI (ADD 5) Holland / cow - caramel crunch, brown butter pork - black truffle, sea salt JAKE’S GOUDA JAMON SERRANO New York / raw cow - addictive, caramelized pineapple pork - salty, lean, gamey ESSEX BRABANDER SMOKED DUCK BREAST Netherlands / goat - sweet, nutty, caramel duck - tender, sweet, gently smoked TOMME DE MA GRAND MERE ANGEL’S BISON SALAMI (ADD 5) France / goat - bright, tangy, -

The Tasty World of Charcuterie
Welcome to the DISCOVER THE FLAVORS Tasty World of Charcuterie IN YOUR CHARCUTERIE BOX Charcuterie (SHär’kooduh-ree) is how the French refer to smoked, cured or cooked meats. This box of charcuterie is packed with exciting flavors you’re sure to enjoy. These traditional methods of preservation were perfected in the days before refrigeration. Our charcuterie collections contain different combinations of the products below.* Bon appétit! You’ve probably already enjoyed charcuterie; ever had bacon, salami or ham? Pork Saucisson Sec: Ready-to-eat dry-cured pork sausage, similar to salami Slice thinly and serve with black truffle butter on bread, or pizza; dice for carbonara MAKING A CHARCUTERIE PLATE Duck Saucisson Sec: Ready-to-eat dry-cured duck sausage with warming spices A charcuterie plate is probably one of the easiest, Slice thinly and serve on charcuterie board; crumble and add to fried rice and most impressive appetizers to serve. 1. On a board or platter, arrange an assortment of ready-to-eat charcuterie. Wild Boar Saucisson Sec: Ready-to-eat dry-cured wild boar sausage, robustly flavored Dice, add to pasta salad; slice thinly for cheese and charcuterie plate 2. Add a variety of complementary sides and condiments. Our recommendations - items likely in your pantry or readily available Black Truffle Butter: Ready-to-eat butter studded with pieces of black truffle at your local grocer - include: Pairs well with eggs, bread, potatoes, pasta or popcorn; add a dollop to a steak before serving; • Something acidic like cornichons/tart pickles, mustard or olives add to a charcuterie plate; pair with saucisson sec on a slice of bread • Something sweet like chutney, raw and/or dried fruits such as figs, grapes or melon Chorizo Sausage: Ready-to-eat fully-cooked heritage-breed pork sausage with a little spice • Sliced rustic bread and/or plain crackers Slice and serve at room temperature on charcuterie board; sauté or grill for paella, soups or stews A hearty red wine always pairs well with a charcuterie plate. -

OF DRY-CURED MEAT PRODUCTS: HIGH PRESSURE and Nacl-FREE PROCESSING IMPLEMENTATION
FOOD SAFETY IN FAST DRYING (QDS PROCESS®) OF DRY-CURED MEAT PRODUCTS: HIGH PRESSURE AND NaCl-FREE PROCESSING IMPLEMENTATION Katharina STOLLEWERK Dipòsit legal: GI. 1725-2012 http://hdl.handle.net/10803/96264 Food safety in fast drying (QDS process®) of dry-cured meat products: high pressure and NaCl-free processing implementation està subjecte a una llicència de Reconeixement-NoComercial-SenseObraDerivada 3.0 No adaptada de Creative Commons PhD Thesis Food safety in fast drying (QDS process®) of dry-cured meat products: High Pressure and NaCl-free processing implementation. Katharina Stollewerk May, 2012 PhD Thesis Food safety in fast drying (QDS process®) of dry-cured meat products: High Pressure and NaCl-free processing implementation. Author: Katharina Stollewerk 2012 PhD Programme in Technology PhD Supervisors: Dr. Josep Comaposada Beringues and Dr. Anna Jofré Fradera Tutor: Dr. Carmen Carretero Romany Dissertation submitted to apply for the Doctor degree by the University of Girona Katharina Stollewerk Dr. Josep Comaposada Dr. Anna Jofré Dr. Carmen Carretero Dr. Josep Comaposada i Beringues, director of the Subprogram New process technologies in the food industry (Food Technology Programme) and Dr. Anna Jofré i Fradera, researcher of the Food Safety Programme of IRTA, El Dr. Josep Comaposada i Beringues, director del Subprograma de noves tecnologies de procés a la indústria alimentària (Programa de tecnologia alimentària) i la Dra. Anna Jofré i Fradera, investigadora del Programa de seguretat alimentària de l’IRTA, CERTIFY: That they have supervised the present PhD thesis entitled “Food safety in fast drying (QDS process®) of dry-cured meat products: High pressure and NaCl-free processing implementation” and presented by Katharina Stollewerk for obtaining the Ph.D. -

Chapter 18 : Sausage the Casing
CHAPTER 18 : SAUSAGE Sausage is any meat that has been comminuted and seasoned. Comminuted means diced, ground, chopped, emulsified or otherwise reduced to minute particles by mechanical means. A simple definition of sausage would be ‘the coarse or finely comminuted meat product prepared from one or more kind of meat or meat by-products, containing various amounts of water, usually seasoned and frequently cured .’ In simplest terms, sausage is ground meat that has been salted for preservation and seasoned to taste. Sausage is one of the oldest forms of charcuterie, and is made almost all over the world in some form or the other. Many sausage recipes and concepts have brought fame to cities and their people. Frankfurters from Frankfurt in Germany, Weiner from Vienna in Austria and Bologna from the town of Bologna in Italy. are all very famous. There are over 1200 varieties world wide Sausage consists of two parts: - the casing - the filling THE CASING Casings are of vital importance in sausage making. Their primary function is that of a holder for the meat mixture. They also have a major effect on the mouth feel (if edible) and appearance. The variety of casings available is broad. These include: natural, collagen, fibrous cellulose and protein lined fibrous cellulose. Some casings are edible and are meant to be eaten with the sausage. Other casings are non edible and are peeled away before eating. 1 NATURAL CASINGS: These are made from the intestines of animals such as hogs, pigs, wild boar, cattle and sheep. The intestine is a very long organ and is ideal for a casing of the sausage. -

Pièces De Porc Séchées
Commande de viande en RHd Pièces de porc FiCHe teCHnique séchées charcuterie Les jambons crus, jambons crus de pays, jambons secs PRODUITS DÉNOMINATIONS/PRÉSENTATIONS CARACTÉRISTIQUES Jambon sec, Entier avec os ou désossé, tranché Sur le produit prêt à consommer jambon sel sec, - jambon sec supérieur - jambon sec traditionnel Critères nutritionnels supérieur - jambon sel sec supérieur Sel (sodium x 2,5) < 6,9 g/100g ou traditionnel - jambon sel sec supérieur traditionnel Critères technologiques Mentions sucres solubles totaux (SST) ≤ 1 % - «à l’ancienne» Le cycle de fabrication, de la mise au sel à la sortie du séchoir, - fumé, est d’au moins 210 jours Egalement Pour la fréquence de distribution - jambon (sel) sec supérieur du Haut Doubs, Lipides < 15% ou > 15% en fonction du produit - jambon (sel) sec supérieur de Luxeuil, P/L > 1 - jambon (sel) sec supérieur de Savoie Jambon sec, Entier avec os ou désossé, tranché Sur le produit prêt à consommer jambon sel sec - jambon sec - jambon sel sec Critères nutritionnels Sel (sodium x 2,5) < 6,9 g/100g Egalement Critères technologiques - jambon sec à, au(x) sucres solubles totaux (SST) ≤ 1 % - jambon sec du Haut Doubs Le cycle de fabrication, de la mise au sel à la sortie du séchoir, - jambon sec de Luxeuil, est d’au moins 130 jours. - jambon sec de Savoie Pour la fréquence de distribution Lipides < 15% ou > 15% en fonction du produit P/L > 1 Jambon cru, Entier avec os ou désossé, tranché Sur le produit prêt à consommer jambon cru de pays - jambon cru, - jambon cru de pays Critères nutritionnels -

Acuerdos Internacionales
24.10.2017 ES Diario Oficial de la Unión Europea L 274/1 II (Actos no legislativos) ACUERDOS INTERNACIONALES DECISIÓN (UE) 2017/1912 DEL CONSEJO de 9 de octubre de 2017 relativa a la celebración del Acuerdo entre la Unión Europea e Islandia sobre la protección de las indicaciones geográficas de los productos agrícolas y alimenticios EL CONSEJO DE LA UNIÓN EUROPEA, Visto el Tratado de Funcionamiento de la Unión Europea, y en particular su artículo 207, apartado 4, párrafo primero, leído en relación con su artículo 218, apartado 6, letra a), inciso v), y su artículo 218, apartado 7, Vista la propuesta de la Comisión Europea, Vista la aprobación del Parlamento Europeo, Considerando lo siguiente: (1) De conformidad con la Decisión (UE) 2016/2136 del Consejo (1), el Acuerdo entre la Unión Europea e Islandia sobre la protección de las indicaciones geográficas de los productos agrícolas y alimenticios (en lo sucesivo, «Acuerdo») fue firmado por la Comisión el 23 de marzo de 2017, a reserva de su celebración en una fecha posterior. (2) Las Partes se comprometen a promover entre sí el desarrollo armonioso de las indicaciones geográficas tal como se define en el artículo 22, apartado 1, del Acuerdo sobre los Aspectos de los Derechos de Propiedad Intelectual relacionados con el Comercio (ADPIC) (2) y a fomentar el comercio de los productos agrícolas y alimenticios con indicaciones geográficas originarios de los territorios de las Partes. (3) En virtud del Acuerdo, aunque Islandia todavía no ha registrado ninguna indicación geográfica protegida, las indicaciones geográficas de los productos agrícolas y alimenticios de la Unión estarán protegidas en Islandia. -

Artisanal Charcutterie
ARTISANAL CHARCUTTERIE LIST OF PRODUCTS & DESCRIPTION Guanciale Guanciale is an Italian cured meat product prepared from pork jowl or cheeks. Its name is derived from guancia, Italian for cheek. 520 THB/KG Its flavor is stronger than other pork products, such as pancetta, and its texture is more delicate. Upon cooking, the fat typically melts away giving great depth of flavor to the dishes and sauces it is used in. In cuisine Guanciale may be cut and eaten directly in small portions, but is often used as a pasta ingredient.It is used in dishes like spaghetti alla carbonara and sauces like sugo all'amatriciana. Coppa / Cabecero de lomo Capocollo or Coppa is a traditional Italian and Corsican pork cold cut (salume) made from the dry-cured muscle running from the neck to the 4th or 5th rib of the pork shoulder or neck. 1030 THB/ Kg It is a whole muscle salume, dry cured and, typically, sliced very thin. It is similar to the more widely known cured ham or prosciutto, because they are both pork-derived cold-cuts that are used in similar dishes This cut is typically called capocollo or coppa in much of Italy and Corsica. Regional terms include capicollo (Campania), capicollu (Corsica), finocchiata (Tuscany), lonza (Lazio) and lonzino (Marche and Abruzzo). Capocollo is esteemed for its delicate flavor and tender, fatty texture and is often more expensive than most other salumi. In many countries, it is often sold as a gourmet food item. It is usually sliced thin for use in antipasto or sandwiches such as muffulettas, Italian grinders and subs, and panini as well as some traditional Italian pizza. -

Iceland on the Protection of Geographical Indications for Agricultural Products and Foodstuffs
24.10.2017 EN Official Journal of the European Union L 274/3 AGREEMENT between the European Union and Iceland on the protection of geographical indications for agricultural products and foodstuffs THE EUROPEAN UNION, of the one part, and ICELAND of the other part, hereinafter referred to as the ‘Parties’, CONSIDERING that the Parties agree to promote between each other a harmonious development of the geographical indications as defined in Article 22(1) of the Agreement on Trade-Related Aspects of Intellectual Property Rights (TRIPS) and to foster trade in agricultural products and foodstuffs originating in the Parties' territories, CONSIDERING that the Agreement on the European Economic Area (EEA Agreement) provides for the mutual recognition and protection of geographical indications of wines, aromatised wine products and spirit drinks, HAVE AGREED AS FOLLOWS: Article 1 Scope 1. This Agreement applies to the recognition and protection of geographical indications for agricultural products and foodstuffs other than wines, aromatised wine products and spirit drinks originating in the Parties' territories. 2. Geographical indications of a Party shall be protected by the other Party under this Agreement only if covered by the scope of the legislation referred to in Article 2. Article 2 Established geographical indications 1. Having examined the legislation of Iceland listed in Part A of Annex I, the European Union concludes that that legislation meets the elements laid down in Part B of Annex I. 2. Having examined the legislation of the European Union listed in Part A of Annex I, Iceland concludes that that legislation meets the elements laid down in Part B of Annex I. -
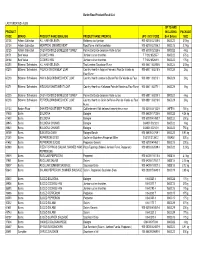
Revised Bartor Product List For
Bartor Road Product Recall List LAST UPDATED: AU25 UP TO AND PRODUCT INCLUDING PACKAGE CODE BRAND PRODUCT NAME (ENGLISH) PRODUCT NAME (FRENCH) UPC / SCC CODE Best Before: SIZE 32138 Artisan Collection ALL HAM KIELBASA Kielbassa tout jambon 900 63100 32138 6 08OC22 3.5 kg 32136 Artisan Collection MONTREAL SMOKED MEAT Boeuf fume a la Montrealaise 900 63100 32136 2 08OC15 2.7 kg 32125 Artisan Collection OVEN ROASTED BONELESS TURKEY Poitine De Dinde desossee Rotie au four 900 63100 32125 6 08OC22 4 kg 24171 Best Value COOKED HAM Jambon cuit en tranches 7 71212 85255 7 08OC22 375 g 38164 Best Value COOKED HAM Jambon cuit en tranches 7 71212 85264 9 08OC22 175 g 60250 Bittners / Schneiders ALL HAM KIELBASA Tout Jambon Saucisson Fume 900 60811 60250 6 08OC22 3.5 kg 60215 Bittners / Schneiders FRENCH ONION MEAT LOAF Country Hearth a l'oignon Francais Pain De Viande au 900 60811 60215 5 08OC29 2 kg Four Fume 60213 Bittners / Schneiders HAM & BACON BAKED MEAT LOAF Country Hearth Jambon & Bacon Pain De Viande au Four 900 60811 60213 1 08OC29 2 kg 60271 Bittners / Schneiders KIELBASA BAKED MEAT LOAF Country Hearth au Kielbassa Pain de Viande au Four Fume 900 60811 60271 1 08OC29 2 kg 60235 Bittners / Schneiders OVEN ROASTED BONELESS TURKEY Poitine De Dinde desossee Rotie au four 900 60811 60235 3 08OC22 4 kg 60218 Bittners / Schneiders PEPPERCORN BAKED MEAT LOAF Country Hearth au Grain de Poivre Pain de Viande au Four 900 60811 60218 6 08OC29 2 kg Fume 07133 Boston Pizza SHAVED ROAST BEEF FROZEN Cuit lentement Rôti de boeuf tranché trés mince 100 -

No 1151/2012 of the European Parliament and of the Council on Quality Schemes for Agricultural Products and Foodstuffs (2015/C 182/07)
3.6.2015 EN Official Journal of the European Union C 182/7 OTHER ACTS EUROPEAN COMMISSION Publication of an application pursuant to Article 50(2)(a) of Regulation (EU) No 1151/2012 of the European Parliament and of the Council on quality schemes for agricultural products and foodstuffs (2015/C 182/07) This publication confers the right to oppose the application pursuant to Article 51 of Regulation (EU) No 1151/2012 of the European Parliament and of the Council (1). SINGLE DOCUMENT ‘SAUCISSON D’ARDENNE’/‘COLLIER D’ARDENNE’/‘PIPE D’ARDENNE’ EU No: BE-PGI-0005-01222 — 08.05.2014 PDO ( ) PGI ( X ) 1. Name ‘Saucisson d’Ardenne’/‘Collier d’Ardenne’/‘Pipe d’Ardenne’ 2. Member State or Third Country Belgium 3. Description of the agricultural product or foodstuff 3.1. Type of product Class 1.2. Meat products (cooked, salted, smoked, etc.) 3.2. Description of the product to which the name in (1) applies The three names refer to the same smoked, dried, cold meat product (sausage), produced by the fermentation of pork, or pork and beef. This cylindrically-shaped product exists in three forms (each with its own name): — ‘saucisson’: long and straight, with a diameter of between 30 and 90 mm, a maximum length of 60 cm (and maximum weight of approximately 2,2 kg) when it is sold to the consumer whole, — ‘collier’: semi-circular, with a diameter of between 20 and 40 mm and a weight of between 200 and 600 g, — ‘pipe’: long and straight, with a weight and diameter of maximum 150 g and 30 mm respectively.