Chemical Engineering Thermodynamics II
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Chapter 4 Calculation of Standard Thermodynamic Properties of Aqueous Electrolytes and Non-Electrolytes
Chapter 4 Calculation of Standard Thermodynamic Properties of Aqueous Electrolytes and Non-Electrolytes Vladimir Majer Laboratoire de Thermodynamique des Solutions et des Polymères Université Blaise Pascal Clermont II / CNRS 63177 Aubière, France Josef Sedlbauer Department of Chemistry Technical University Liberec 46117 Liberec, Czech Republic Robert H. Wood Department of Chemistry and Biochemistry University of Delaware Newark, DE 19716, USA 4.1 Introduction Thermodynamic modeling is important for understanding and predicting phase and chemical equilibria in industrial and natural aqueous systems at elevated temperatures and pressures. Such systems contain a variety of organic and inorganic solutes ranging from apolar nonelectrolytes to strong electrolytes; temperature and pressure strongly affect speciation of solutes that are encountered in molecular or ionic forms, or as ion pairs or complexes. Properties related to the Gibbs energy, such as thermodynamic equilibrium constants of hydrothermal reactions and activity coefficients of aqueous species, are required for practical use by geologists, power-cycle chemists and process engineers. Derivative properties (enthalpy, heat capacity and volume), which can be obtained from calorimetric and volumetric experiments, are useful in extrapolations when calculating the Gibbs energy at conditions remote from ambient. They also sensitively indicate evolution in molecular interactions with changing temperature and pressure. In this context, models with a sound theoretical basis are indispensable, describing with a limited number of adjustable parameters all thermodynamic functions of an aqueous system over a wide range of temperature and pressure. In thermodynamics of hydrothermal solutions, the unsymmetric standard-state convention is generally used; in this case, the standard thermodynamic properties (STP) of a solute reflect its interaction with the solvent (water), and the excess properties, related to activity coefficients, correspond to solute-solute interactions. -

Chemistry Courses 2005-2006
Chemistry Courses 2005-2006 Autumn 2005 Chem 11101 General Chemistry I, Variant A Lee Chem 11102 General Chemistry I, Variant B Norris Chem 12200 Honors General Chemistry I Levy Chem 22000 Organic Chemistry I Yu Chem 22300 Intermediate Organic Chemistry Mrksich Chem 26100 Quantum Mechanics Mazziotti Chem 30100 Advanced Inorganic Chemistry Hopkins Chem 30900 Bioinorganic Chemistry He Chem 32100 Physical Organic Chemistry I Ismagilov Chem 32200 Organic Synthesis and Structure Rawal Chem 32600 Protein Fundamentals Piccirilli Chem 36100 Wave Mechanics & Spectroscopy Butler Chem 36400 Chemical Thermodynamics Dinner Winter 2006 Chem 11201 General Chemistry II, Variant A Scherer Chem 11202 General Chemistry II, Variant B Butler Chem 12300 Honors General Chemistry II Dinner Chem 20100 Inorganic Chemistry I Hillhouse Chem 22100 Organic Chemistry II Rawal Chem 23100 Honors Organic Chemistry II Kozmin Chem 26200 Thermodynamics Norris Chem 26700 Experimental Physical Chemistry Levy Chem 30200 Synthesis & Physical Methods in Inorganic Chemistry Jordan Chem 30400 Organometallic Chemistry Bosnich Chem 32300 Tactics of Organic Synthesis Yamamoto Chem 32400 Physical Organic Chemistry II Mrksich Chem 36200 Quantum Mechanics Freed Chem 36300 Statistical Mechanics Mazziotti Chem 38700 Biophysical Chemistry Lee Spring 2006 Chem 11301 General Chemistry III, Variant A Kozmin Chem 11302 General Chemistry III, Variant B Guyot-Sionnest Chem 20200 Inorganic Chemistry II Jordan Chem 22200 Organic Chemistry III Kent Chem 23200 Honors Organic Chemistry III Yamamoto Chem 22700 Advanced Organic / Inorganic Laboratory (8 students) He Chem 26300 Chemical Kinetics and Dynamics Sibner Chem 26800 Computational Chemistry & Biology Freed Chem 30600 Chemistry of the Elements Hillhouse Chem 31100 Supramolecular Chemistry Bosnich Chem 32500 Bioorganic Chemistry Piccirilli Chem 32900 Polymer Chemistry Yu Chem 33000 Complex Systems Ismagilov Chem 36500 Chemical Dynamics Scherer Chem 36800 Advanced Computational Chemistry & Biology Freed . -
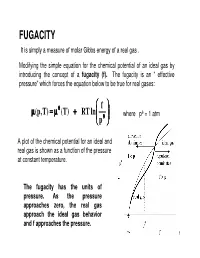
FUGACITY It Is Simply a Measure of Molar Gibbs Energy of a Real Gas
FUGACITY It is simply a measure of molar Gibbs energy of a real gas . Modifying the simple equation for the chemical potential of an ideal gas by introducing the concept of a fugacity (f). The fugacity is an “ effective pressure” which forces the equation below to be true for real gases: θθθ f µµµ ,p( T) === µµµ (T) +++ RT ln where pθ = 1 atm pθθθ A plot of the chemical potential for an ideal and real gas is shown as a function of the pressure at constant temperature. The fugacity has the units of pressure. As the pressure approaches zero, the real gas approach the ideal gas behavior and f approaches the pressure. 1 If fugacity is an “effective pressure” i.e, the pressure that gives the right value for the chemical potential of a real gas. So, the only way we can get a value for it and hence for µµµ is from the gas pressure. Thus we must find the relation between the effective pressure f and the measured pressure p. let f = φ p φ is defined as the fugacity coefficient. φφφ is the “fudge factor” that modifies the actual measured pressure to give the true chemical potential of the real gas. By introducing φ we have just put off finding f directly. Thus, now we have to find φ. Substituting for φφφ in the above equation gives: p µ=µ+(p,T)θ (T) RT ln + RT ln φ=µ (ideal gas) + RT ln φ pθ µµµ(p,T) −−− µµµ(ideal gas ) === RT ln φφφ This equation shows that the difference in chemical potential between the real and ideal gas lies in the term RT ln φφφ.φ This is the term due to molecular interaction effects. -

Entropy and Life
Entropy and life Research concerning the relationship between the thermodynamics textbook, states, after speaking about thermodynamic quantity entropy and the evolution of the laws of the physical world, that “there are none that life began around the turn of the 20th century. In 1910, are established on a firmer basis than the two general American historian Henry Adams printed and distributed propositions of Joule and Carnot; which constitute the to university libraries and history professors the small vol- fundamental laws of our subject.” McCulloch then goes ume A Letter to American Teachers of History proposing on to show that these two laws may be combined in a sin- a theory of history based on the second law of thermo- gle expression as follows: dynamics and on the principle of entropy.[1][2] The 1944 book What is Life? by Nobel-laureate physicist Erwin Schrödinger stimulated research in the field. In his book, Z dQ Schrödinger originally stated that life feeds on negative S = entropy, or negentropy as it is sometimes called, but in a τ later edition corrected himself in response to complaints and stated the true source is free energy. More recent where work has restricted the discussion to Gibbs free energy because biological processes on Earth normally occur at S = entropy a constant temperature and pressure, such as in the atmo- dQ = a differential amount of heat sphere or at the bottom of an ocean, but not across both passed into a thermodynamic sys- over short periods of time for individual organisms. tem τ = absolute temperature 1 Origin McCulloch then declares that the applications of these two laws, i.e. -

Chapter 8 Thermodynamic Properties of Mixtures
Chapter 8 Thermodynamic Properties of Mixtures 2012/3/29 1 Abstract The thermodynamic description of mixtures, extended from pure fluids. The equations of change, i.e., energy and entropy balance, for mixtures are developed. The criteria for phase and chemical equilibrium in mixtures 2012/3/29 2 8.1 THE THERMODYNAMIC DESCRIPTION OF MIXTURES Thermodynamic property for pure fluids, θθ=()TPN , , where N is the number of moles. θθ=()TP , where the number of mole equals to 1. Thermodynamic property for mixtures, θθ=()TPN , ,12 , N ,L , Nc where Ni is the number of moles of the ith component. θθ=()TPx , ,12 , x ,L , xci where x is the mole fraction of the ith component. For example UUTPNN=() , ,12 , ,LL , Ncc or UUTPxx=() , , 12 , , , x VVTPNN=() , ,12 , ,L , Nc or VVTPxx=() , ,12 , ,L , xc 2012/3/29 3 Summation of the properties of pure fluids (before mixing at TP and ) C UTPxx(), ,12 , ,L , xci− 1= ∑ xUTPi () , (8.1-1) i=1 where UU is the molar internal energy, i is the internal energy of the pure i-th component at TP and . C ˆˆ UTPww()(), ,12 , ,L , wcii− 1= ∑ wUTP , (8.1-2) i=1 where wi is the mass fraction of component i. 2012/3/29 4 At the same T and P 50 cc 25 cc + 25 cc H2O H2O or 52 cc 25 cc + 25 cc 48 cc + 2 cc A B -2 cc Attractive Repulsive 2012/3/29 5 Property change upon mixing (at constantTP and ) C Δ=mixθθ()TPx,,ii −∑ x θi () TP , i=1 Volume change upon mixing C Δ=mixVTP(),,,, VTPx()ii −∑ xVTPi () i=1 Enthalpy change upon mixing C Δ=mix HTP(),,,, HTPx()ii −∑ xHTPi () i=1 2012/3/29 6 Experimental data : properties changes upon mixing (H and V) Figure 8.1-1 Enthalpy-concentration diagram for aqueous sulfuric acid at 0.1 MPa. -

Surface Tension Study of Concentration Dependent Cluster Breaking in Acetone-Alcohol Systems
www.sciencevision.org Sci Vis 12 (3), 102-105 July-September 2012 Original Research ISSN (print) 0975-6175 ISSN (online) 2229-6026 Surface tension study of concentration dependent cluster breaking in acetone-alcohol systems Jonathan Lalnunsiama* and V. Madhurima Department of Physics, Mizoram University, Aizawl 796 004, India Received 19 April 2012 | Revised 10 May 2012 | Accepted 13 August 2012 ABSTRACT Alcohols are well-known for the formation of clusters due to hydrogen bonds. When a second mo- lecular species such as acetone is added to an alcohol system, the hydrogen bonds are broken lead- ing to a destruction of the molecular clusters. In this paper we report the findings of surface ten- sion study over the entire concentration region of acetone and six alcohol systems over the entire concentration range. The alcohols chosen were methanol, ethanol, isopropanol, butanol, hexanol and octanol. Surface tension was measured using the pendent drop method. Our study showed a specific molecular interaction near the 1:1 concentration of lower alcohols and acetone whereas the higher alcohols did not exhibit the same. Key words: Surface tension; hydrogen bond; alcohols; acetone. INTRODUCTION vent effects4 but such effects are important and hence there are many studies of hydrogen Alcohols are marked by their ability to bonds in solvents.5 Dielectric and calorimetric form hydrogen bonded clusters.1,2 The addi- studies of clusters in alcohols in carbon-tertra- tion of a second molecular species, whether chloride were attributed to homogeneous as- hydrogen-bonded or not, will change the in- sociations of the alcohol.6 For methanol in p- termolecular interactions in the alcohol sys- dioxane the chain-like clusters were seen to tem, especially by breaking of the hydrogen- form the networks and not cyclic structures.7 bonded clusters. -

Answer Key Chapter 16: Standard Review Worksheet – + 1
Answer Key Chapter 16: Standard Review Worksheet – + 1. H3PO4(aq) + H2O(l) H2PO4 (aq) + H3O (aq) + + NH4 (aq) + H2O(l) NH3(aq) + H3O (aq) 2. When we say that acetic acid is a weak acid, we can take either of two points of view. Usually we say that acetic acid is a weak acid because it doesn’t ionize very much when dissolved in water. We say that not very many acetic acid molecules dissociate. However, we can describe this situation from another point of view. We could say that the reason acetic acid doesn’t dissociate much when we dissolve it in water is because the acetate ion (the conjugate base of acetic acid) is extremely effective at holding onto protons and specifically is better at holding onto protons than water is in attracting them. + – HC2H3O2 + H2O H3O + C2H3O2 Now what would happen if we had a source of free acetate ions (for example, sodium acetate) and placed them into water? Since acetate ion is better at attracting protons than is water, the acetate ions would pull protons out of water molecules, leaving hydroxide ions. That is, – – C2H3O2 + H2O HC2H3O2 + OH Since an increase in hydroxide ion concentration would take place in the solution, the solution would be basic. Because acetic acid is a weak acid, the acetate ion is a base in aqueous solution. – – 3. Since HCl, HNO3, and HClO4 are all strong acids, we know that their anions (Cl , NO3 , – and ClO4 ) must be very weak bases and that solutions of the sodium salts of these anions would not be basic. -

An Entropy-Stable Hybrid Scheme for Simulations of Transcritical Real-Fluid
Journal of Computational Physics 340 (2017) 330–357 Contents lists available at ScienceDirect Journal of Computational Physics www.elsevier.com/locate/jcp An entropy-stable hybrid scheme for simulations of transcritical real-fluid flows Peter C. Ma, Yu Lv ∗, Matthias Ihme Department of Mechanical Engineering, Stanford University, Stanford, CA 94305, USA a r t i c l e i n f o a b s t r a c t Article history: A finite-volume method is developed for simulating the mixing of turbulent flows at Received 30 August 2016 transcritical conditions. Spurious pressure oscillations associated with fully conservative Received in revised form 9 March 2017 formulations are addressed by extending a double-flux model to real-fluid equations of Accepted 12 March 2017 state. An entropy-stable formulation that combines high-order non-dissipative and low- Available online 22 March 2017 order dissipative finite-volume schemes is proposed to preserve the physical realizability Keywords: of numerical solutions across large density gradients. Convexity conditions and constraints Real fluid on the application of the cubic state equation to transcritical flows are investigated, and Spurious pressure oscillations conservation properties relevant to the double-flux model are examined. The resulting Double-flux model method is applied to a series of test cases to demonstrate the capability in simulations Entropy stable of problems that are relevant for multi-species transcritical real-fluid flows. Hybrid scheme © 2017 Elsevier Inc. All rights reserved. 1. Introduction The accurate and robust simulation of transcritical real-fluid effects is crucial for many engineering applications, such as fuel injection in internal-combustion engines, rocket motors and gas turbines. -

Material Safety Data Sheet Ethanol SDA1, Anhydrous MSDS# 88067 Section 1
Material Safety Data Sheet Ethanol SDA1, Anhydrous MSDS# 88067 Section 1 - Chemical Product and Company Identification MSDS Name: Ethanol SDA1, Anhydrous Catalog Numbers: A405-20, A405P-4 Synonyms: Ethyl alcohol, denatured; Grain alcohol, denatured; Ethyl hydroxide, denatured. Fisher Scientific Company Identification: One Reagent Lane Fair Lawn, NJ 07410 For information in the US, call: 201-796-7100 Emergency Number US: 201-796-7100 CHEMTREC Phone Number, US: 800-424-9300 Section 2 - Composition, Information on Ingredients ---------------------------------------- Risk Phrases: 11 CAS#: 64-17-5 Chemical Name: Ethyl alcohol %: 92-93 EINECS#: 200-578-6 Hazard Symbols: F ---------------------------------------- ---------------------------------------- Risk Phrases: 11 23/24/25 39/23/24/25 CAS#: 67-56-1 Chemical Name: Methyl alcohol %: 3.7 EINECS#: 200-659-6 Hazard Symbols: F T ---------------------------------------- ---------------------------------------- Risk Phrases: CAS#: 108-10-1 Chemical Name: Methyl iso-butyl ketone %: 1.0-2.0 EINECS#: 203-550-1 Hazard Symbols: ---------------------------------------- ---------------------------------------- Risk Phrases: 11 20 CAS#: 108-88-3 Chemical Name: Toluene %: 0.07 EINECS#: 203-625-9 Hazard Symbols: F XN ---------------------------------------- ---------------------------------------- Risk Phrases: 11 36 66 67 CAS#: 141-78-6 Chemical Name: Ethyl acetate %: <1.0 EINECS#: 205-500-4 Hazard Symbols: F XI ---------------------------------------- Text for R-phrases: see Section 16 Hazard Symbols: XN F Risk Phrases: 11 20/21/22 68/20/21/22 Section 3 - Hazards Identification EMERGENCY OVERVIEW Warning! Flammable liquid and vapor. Causes respiratory tract irritation. May cause central nervous system depression. Causes severe eye irritation. This substance has caused adverse reproductive and fetal effects in humans. Causes moderate skin irritation. May cause liver, kidney and heart damage. Target Organs: Kidneys, heart, central nervous system, liver, eyes, optic nerve. -

Selection of Thermodynamic Methods
P & I Design Ltd Process Instrumentation Consultancy & Design 2 Reed Street, Gladstone Industrial Estate, Thornaby, TS17 7AF, United Kingdom. Tel. +44 (0) 1642 617444 Fax. +44 (0) 1642 616447 Web Site: www.pidesign.co.uk PROCESS MODELLING SELECTION OF THERMODYNAMIC METHODS by John E. Edwards [email protected] MNL031B 10/08 PAGE 1 OF 38 Process Modelling Selection of Thermodynamic Methods Contents 1.0 Introduction 2.0 Thermodynamic Fundamentals 2.1 Thermodynamic Energies 2.2 Gibbs Phase Rule 2.3 Enthalpy 2.4 Thermodynamics of Real Processes 3.0 System Phases 3.1 Single Phase Gas 3.2 Liquid Phase 3.3 Vapour liquid equilibrium 4.0 Chemical Reactions 4.1 Reaction Chemistry 4.2 Reaction Chemistry Applied 5.0 Summary Appendices I Enthalpy Calculations in CHEMCAD II Thermodynamic Model Synopsis – Vapor Liquid Equilibrium III Thermodynamic Model Selection – Application Tables IV K Model – Henry’s Law Review V Inert Gases and Infinitely Dilute Solutions VI Post Combustion Carbon Capture Thermodynamics VII Thermodynamic Guidance Note VIII Prediction of Physical Properties Figures 1 Ideal Solution Txy Diagram 2 Enthalpy Isobar 3 Thermodynamic Phases 4 van der Waals Equation of State 5 Relative Volatility in VLE Diagram 6 Azeotrope γ Value in VLE Diagram 7 VLE Diagram and Convergence Effects 8 CHEMCAD K and H Values Wizard 9 Thermodynamic Model Decision Tree 10 K Value and Enthalpy Models Selection Basis PAGE 2 OF 38 MNL 031B Issued November 2008, Prepared by J.E.Edwards of P & I Design Ltd, Teesside, UK www.pidesign.co.uk Process Modelling Selection of Thermodynamic Methods References 1. -

Drugs and Acid Dissociation Constants Ionisation of Drug Molecules Most Drugs Ionise in Aqueous Solution.1 They Are Weak Acids Or Weak Bases
Drugs and acid dissociation constants Ionisation of drug molecules Most drugs ionise in aqueous solution.1 They are weak acids or weak bases. Those that are weak acids ionise in water to give acidic solutions while those that are weak bases ionise to give basic solutions. Drug molecules that are weak acids Drug molecules that are weak bases where, HA = acid (the drug molecule) where, B = base (the drug molecule) H2O = base H2O = acid A− = conjugate base (the drug anion) OH− = conjugate base (the drug anion) + + H3O = conjugate acid BH = conjugate acid Acid dissociation constant, Ka For a drug molecule that is a weak acid The equilibrium constant for this ionisation is given by the equation + − where [H3O ], [A ], [HA] and [H2O] are the concentrations at equilibrium. In a dilute solution the concentration of water is to all intents and purposes constant. So the equation is simplified to: where Ka is the acid dissociation constant for the weak acid + + Also, H3O is often written simply as H and the equation for Ka is usually written as: Values for Ka are extremely small and, therefore, pKa values are given (similar to the reason pH is used rather than [H+]. The relationship between pKa and pH is given by the Henderson–Hasselbalch equation: or This relationship is important when determining pKa values from pH measurements. Base dissociation constant, Kb For a drug molecule that is a weak base: 1 Ionisation of drug molecules. 1 Following the same logic as for deriving Ka, base dissociation constant, Kb, is given by: and Ionisation of water Water ionises very slightly. -

TSCA Inventory Representation for Products Containing Two Or More
mixtures.txt TOXIC SUBSTANCES CONTROL ACT INVENTORY REPRESENTATION FOR PRODUCTS CONTAINING TWO OR MORE SUBSTANCES: FORMULATED AND STATUTORY MIXTURES I. Introduction This paper explains the conventions that are applied to listings of certain mixtures for the Chemical Substance Inventory that is maintained by the U.S. Environmental Protection Agency (EPA) under the Toxic Substances Control Act (TSCA). This paper discusses the Inventory representation of mixtures of substances that do not react together (i.e., formulated mixtures) as well as those combinations that are formed during certain manufacturing activities and are designated as mixtures by the Agency (i.e., statutory mixtures). Complex reaction products are covered in a separate paper. The Agency's goal in developing this paper is to make it easier for the users of the Inventory to interpret Inventory listings and to understand how new mixtures would be identified for Inventory inclusion. Fundamental to the Inventory as a whole is the principle that entries on the Inventory are identified as precisely as possible for the commercial chemical substance, as reported by the submitter. Substances that are chemically indistinguishable, or even identical, may be listed differently on the Inventory, depending on the degree of knowledge that the submitters possess and report about such substances, as well as how submitters intend to represent the chemical identities to the Agency and to customers. Although these chemically indistinguishable substances are named differently on the Inventory, this is not a "nomenclature" issue, but an issue of substance representation. Submitters should be aware that their choice for substance representation plays an important role in the Agency's determination of how the substance will be listed on the Inventory.