Rosten, Lyn, K. True, E. Wiseman, K
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

DNA-Based Environmental Monitoring for the Invasive Myxozoan Parasite, Myxobolus Cerebralis, in Alberta, Canada
! ! ! ! "#$%&'()*!+,-./0,1),2'3!40,.20/.,5!60/!27)!!8,-'(.-)!49:0;0',!<'/'(.2)=!!"#$%$&'() *+,+%,-&.(=!.,!$3>)/2'=!?','*'! ! >9! ! "',.)33)!+/.,!&'//9! ! ! ! ! ! ! ! ! $!27)(.(!(@>1.22)*!.,!A'/2.'[email protected]),2!06!27)!/)B@./)1),2(!60/!27)!*)5/))!06! ! ! 4'(2)/!06!CD.),D)! ! .,! ! +,-./0,1),2'3!E)'327!CD.),D)(! ! ! ! ! ! CD7003!06!<@>3.D!E)'327! F,.-)/(.29!06!$3>)/2'! ! ! ! ! ! ! ! ! ! ! ! G!"',.)33)!+/.,!&'//9=!HIHI! !! ! ! ! ! ! !"#$%&'$( ! J7./3.,5!*.()'()!.(!'!*.()'()!06!6.(7!D'@()*!>9!',!.,-'(.-)!19:0(A0/)',!A'/'(.2)=! !"#$%$&'()*+,+%,-&.(K!82!L'(!6./(2!*)2)D2)*!.,!?','*'!.,!M07,(0,!N'O)!.,!&',66!#'2.0,'3!<'/O=! $3>)/2'=!.,!$@5@(2!HIPQ=!',*!3.223)!.(!O,0L,!'>0@2!27)!2/',(1.((.0,!06!27.(!A'/'(.2)!.,!?','*'K! ?@//),2!2)(2.,5!60D@()(!0,!27)!*)2)D2.0,!06!!/)*+,+%,-&.(!.,!6.(7!2.((@)(=!/)B@./.,5!3)27'3!2)(2.,5!06! >027!.,6)D2)*!',*!,0,%.,6)D2)*!6.(7K!E0L)-)/=!27)!A'/'(.2)!7'(!'!*)6.,.2.-)!70(2=!27)!03.50D7')2)! L0/1!0'%.1+#)2'%.1+#!',*!2L0!),-./0,1),2'3!(2'5)(!60@,*!.,!L'2)/!',*!()*.1),2!27'2!D/)'2)! 027)/!'-),@)(!60/!*)2)D2.0,K!J)!A/0A0()!27'2!@(.,5!27)!A'/'(.2)!(2'5)(!60@,*!.,!L'2)/!',*! ()*.1),2!',*!27)!'32)/,'2)!L0/1!70(2=!0'%.1+#)2'%.1+#3!'/)!'!/)'(0,'>3)!D01A3)1),2!20!6.(7! ('1A3.,5!',*!L.33!>)!)(A)D.'339!@()6@3!60/!('1A3.,5!.,!'/)'(!L7)/)!6.(7!D033)D2.0,!.(!D7'33),5.,5! 0/!A/07.>.2.-)!*@)!20!-@3,)/'>.3.29!06!27)!6.(7!A0A@3'2.0,(K!8,!'**.2.0,=!0/)2'%.1+#!(@(D)A2.>.3.29!20! !/)*+,+%,-&.(!.(!,02!D0,(.(2),2!'D/0((!27)!(A)D.)(=!L.27!):A)/.1),2(!(70L.,5!(01)!'/)!/)6/'D20/9K! ?7'/'D2)/.;'2.0,!06!27)()!L0/1!A0A@3'2.0,(!L.33!7)3A!2'/5)2!6@2@/)!10,.20/.,5!',*!D0,2/03! -

Area Adventure Hat Creek Ranger District Lassen National Forest
Area Adventure Hat Creek Ranger District Lassen National Forest Welcome The following list of recreation activities are avail- able in the Hat Creek Recreation Area. For more detailed information please stop by the Old Station Visitor Information Center, open April - December, or our District Office located in Fall River Mills. Give Hat Creek Rim Overlook - Nearly 1 million years us a call year-around Mon.- Fri. at (530) 336-5521. ago, active faulting gradually dropped a block of Enjoy your visit to this very interesting country. the Earth’s crust (now Hat Creek Valley) 1,000 feet below the top of the Hat Creek Rim, leaving behind Subway Cave - See an underground cave formed this large fault scarp. This fault system is still “alive by flowing lava. Located just off Highway 89, 1/4 and cracking”. mile north of Old Station junction with Highway 44. The lava tube tour is self guided and the walk is A heritage of the Hat Creek area’s past, it offers mag- 1/3 mile long. Bring a lantern or strong flashlight nificent views of Hat Creek Valley, Lassen Peak, as the cave is not lighted. Sturdy Shoes and a light Burney Mountain, and, further away, Mt. Shasta. jacket are advisable. Subway Cave is closed during the winter months. Fault Hat Creek Rim Fault Scarp Vertical movement Hat Creek V Cross Section of a Lava Tube along this fault system alley dropped this block of earth into its present position Spattercone Trail - Walk a nature trail where volca- nic spattercones and other interesting geologic fea- tures may be seen. -

Comment on G Marty Dcoument
Critique of the Document “Information Regarding Concerns about Farmed Salmon - Wild Salmon Interactions” Presented to the Provincial Government of British Columbia by Gary Marty, D.V.M., Ph.D., Diplomate, A.C.V.P. of the British Columbia Ministry of Agriculture, Animal Health Centre, Abbotsford. Authors of this critique: Lawrence M. Dill1, Martin Krkosek2, Brendan Connors3, Stephanie J. Peacock4, Andrew W. Bateman5, Richard Routledge6, Mark A. Lewis7, and John Reynolds8 1 Professor Emeritus, Department of Biological Sciences, Simon Fraser University 2 Assistant Professor, Department of Ecology and Evolutionary Biology, University of Toronto 3 Senior Systems Ecologist, ESSA Technologies, and Adjunct Professor, Department of Biological Sciences, Simon Fraser University 4 PhD Candidate, Department of Biological Sciences, University of Alberta 5 Postdoctoral Fellow, Department of Biological Sciences, University of Alberta and Department of Ecology and Evolutionary Biology, University of Toronto 6 Professor, Department of Statistics and Actuarial Science, Simon Fraser University 7 Professor and Senior Canada Research Chair, Departments of Biological Sciences and Mathematical and Statistical Sciences, University of Alberta 8 Professor and Tom Buell BC Leadership Chair in Aquatic Conservation, Department of Biological Sciences, Simon Fraser University Background The document, “Information Regarding Concerns about Farmed Salmon - Wild Salmon Interactions,” dated March 16, 2015, was presented to Ministers Thompson and Letnik of the Government of British Columbia (BC) with the intention of providing scientific information upon which to base management and policy decisions regarding wild and farmed salmon in British Columbia. Collectively, we are a group of scientists, mostly academic, whose research expertise includes salmon and infectious diseases (here we refer to infectious diseases in the broadest sense as those that may arise from parasitic, viral or bacterial pathogens). -

Cumulative Factors Potentially Impacting Wild Salmon Declines Kristina M
Evolutionary Applications Evolutionary Applications ISSN 1752-4571 REVIEWS AND SYNTHESIS Infectious disease, shifting climates, and opportunistic predators: cumulative factors potentially impacting wild salmon declines Kristina M. Miller,1,2 Amy Teffer,3 Strahan Tucker,1 Shaorong Li,1 Angela D. Schulze,1 Marc Trudel,1,3 Francis Juanes,3 Amy Tabata,1 Karia H. Kaukinen,1 Norma G. Ginther,1 Tobi J. Ming,1 Steven J. Cooke,6 J. Mark Hipfner,5 David A. Patterson4 and Scott G. Hinch2 1 Pacific Biological Station, Fisheries and Oceans Canada, Nanaimo, BC, Canada 2 Forest and Conservation Sciences, University of British Columbia, Vancouver, BC, Canada 3 Biology Department, University of Victoria, Victoria, BC, Canada 4 Fisheries and Oceans Canada, School of Resource and Environmental Management, Simon Fraser University, Science Branch, Burnaby, BC, Canada 5 Environment Canada, Wildlife Research Division, Delta, BC, Canada 6 Fish Ecology and Conservation Physiology Laboratory, Department of Biology, Carleton Univerisy, Ottawa, ON, Canada Keywords Abstract climate, coevolution, cumulative impacts, ecological impacts, infectious disease, Emerging diseases are impacting animals under high-density culture, yet few microparasite, predation, wild salmon studies assess their importance to wild populations. Microparasites selected for enhanced virulence in culture settings should be less successful maintaining infec- Correspondence tivity in wild populations, as once the host dies, there are limited opportunities Kristina M. Miller, Pacific Biological Station, -

Distribution and Coinfection of Microparasites and Macroparasites in Juvenile Salmonids in Three Upper Willamette River Tributaries
AN ABSTRACT OF THE THESIS OF Sean Robert Roon for the degree of Master of Science in Microbiology presented on December 9, 2014. Title: Distribution and Coinfection of Microparasites and Macroparasites in Juvenile Salmonids in Three Upper Willamette River Tributaries. Abstract approved: ______________________________________________________ Jerri L. Bartholomew Wild fish populations are typically infected with a variety of micro- and macroparasites that may affect fitness and survival, however, there is little published information on parasite distribution in wild juvenile salmonids in three upper tributaries of the Willamette River, OR. The objectives of this survey were to document (1) the distribution of select microparasites in wild salmonids and (2) the prevalence, geographical distribution, and community composition of metazoan parasites infecting these fish. From 2011-2013, I surveyed 279 Chinook salmon Oncorhynchus tshawytscha and 149 rainbow trout O. mykiss for one viral (IHNV) and four bacterial (Aeromonas salmonicida, Flavobacterium columnare, Flavobacterium psychrophilum, and Renibacterium salmoninarum) microparasites known to cause mortality of fish in Willamette River hatcheries. The only microparasite detected was Renibacterium salmoninarum, causative agent of bacterial kidney disease, which was detected at all three sites. I identified 23 metazoan parasite taxa in these fish. Nonmetric multidimensional scaling of metazoan parasite communities reflected a nested structure with trematode metacercariae being the basal parasite taxa at all three sites. The freshwater trematode Nanophyetus salmincola was the most common macroparasite observed at three sites. Metacercariae of N. salmincola have been shown to impair immune function and disease resistance in saltwater. To investigate if N. salmincola affects disease susceptibility in freshwater, I conducted a series of disease challenges to evaluate whether encysted N. -
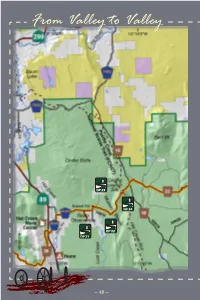
From Valley to Valley
From Valley to Valley DP 23 DP 24 DP 22 DP 21 ~ 48 ~ Emigration in Earnest DP 25 ~ 49 ~ Section 5, Emigration in Earnest ValleyFrom to Valley Emigration in Earnest Section 5 Discovery Points 21 ~ 25 Distance ~ 21.7 miles eventually developed coincides he valleys of this region closely to the SR 44 Twere major thoroughfares for route today. the deluge of emigrants in the In 1848, Peter Lassen and a small 19th century. Linking vale to party set out to blaze a new trail dell, using rivers as high-speed into the Sacramento Valley and to transit, these pioneers were his ranch near Deer Creek. They intensely focused on finding the got lost, but were eventually able quickest route to the bullion of to join up with other gold seekers the Sacramento Valley. From and find a route to his land. His trail became known as the “Death valley to valley, this land Route” and was abandoned within remembers an earnest two years. emigration. Mapquest, circa 1800 During the 1800s, Hat Creek served as a southern “cut-off” from the Pit River allowing emigrants to travel southwest into the Sacramento Valley. Imagine their dismay upon reaching the Hat Creek Rim with the valley floor 900 feet below! This escarpment was caused by opposite sides of a fracture, leaving behind a vertical fault much too steep for the oxen teams and their wagons to negotiate. The path that was Photo of Peter Lassen, courtesy of the Lassen County Historical Society Section 5, Emigration in Earnest ~ 50 ~ Settlement in Fall River and Big Valley also began to take shape during this time. -

California Department of Forestry and Fire Protection Cal Fire
CALIFORNIA DEPARTMENT OF FORESTRY AND FIRE PROTECTION CAL FIRE SHASTA – TRINITY UNIT FIRE PLAN Community Wildfire Protection Plan Mike Chuchel Unit Chief Scott McDonald Division Chief – Special Operations Mike Birondo Battalion Chief - Prevention Bureau Kimberly DeSena Fire Captain – Pre Fire Engineering 2008 Shasta – Trinity Unit Fire Plan 1 Table of Contents 1. EXECUTIVE SUMMARY.................................................................... 4 Unit Fire Plan Assessments and Data Layers................................................ 5 Fire Plan Applications...................................................................................... 6 Community Wildfire Protection Plan............................................................. 6 Unit Fire Plan Responsibilities........................................................................ 6 Key Issues .......................................................................................................... 7 2. STAKEHOLDERS................................................................................. 8 Fire Safe Organizations.................................................................................... 8 Resource Conservation Districts..................................................................... 9 Watershed Contact List ................................................................................... 9 Government Agencies..................................................................................... 13 3. UNIT OVERVIEW ............................................................................. -

Assessment of the Risk to Norwegian Biodiversity and Aquaculture from Pink Salmon
VKM Report 2020: 01 Assessment of the risk to Norwegian biodiversity and aquaculture from pink salmon (Oncorhynchus gorbuscha) Scientific Opinion of the Panel on Alien Organisms and Trade in Endangered Species of the Norwegian Scientific Committee for Food and Environment Report from the Norwegian Scientific Committee for Food and Environment (VKM) 2020: 01 Assessment of the risk to Norwegian biodiversity and aquaculture from pink salmon (Oncorhynchus gorbuscha). Scientific Opinion of the Panel on Alien Organisms and Trade in Endangered Species (CITES) of the Norwegian Scientific Committee for Food and Environment. 15.01.2020 ISBN: 978-82-8259-334-2 ISSN: 2535-4019 Norwegian Scientific Committee for Food and Environment (VKM) Po 222 Skøyen N – 0213 Oslo Norway Phone: +47 21 62 28 00 Email: [email protected] vkm.no vkm.no/english Cover photo: Colourbox Suggested citation: VKM, Kjetil Hindar, Lars Robert Hole, Kyrre Kausrud, Martin Malmstrøm, Espen Rimstad, Lucy Robertson, Odd Terje Sandlund, Eva B. Thorstad, Knut Wiik Vollset, Hugo de Boer, Katrine Eldegard, Johanna Järnegren, Lawrence Kirkendall, Inger Måren, Anders Nielsen, Erlend B. Nilsen, Eli Rueness and Gaute Velle (2020). Assessment of the risk to Norwegian biodiversity and aquaculture from pink salmon (Oncorhynchus gorbuscha). Scientific Opinion of the Panel on Alien Organisms and Trade in Endangered Species (CITES). VKM report 2020:01, ISBN: 978-82-8259-334-2, ISSN: 2535-4019. Norwegian Scientific Committee for Food and Environment (VKM), Oslo, Norway. VKM Report 2020: 01 Assessment of the risk to Norwegian biodiversity and aquaculture from pink salmon (Oncorhynchus gorbuscha) Preparation of the opinion The Norwegian Scientific Committee for Food and Environment (Vitenskapskomiteen for mat og miljø, VKM) appointed a project group to ansver the mandate. -

AFRREV STECH, Vol. 1 (3) August-December, 2012
AFRREV STECH, Vol. 1 (3) August-December, 2012 AFRREV STECH An International Journal of Science and Technology Bahir Dar, Ethiopia Vol.1 (3) August-December, 2012:231-252 ISSN 2225-8612 (Print) ISSN 2227-5444 (Online) Prevalence of Henneguya Chrysichthys and Its Infection Effect on Chrysichthys Nigrodigitatus Fecundity Abraham, J.T and Akpan, P.A Department of Biological Sciences Cross River University of Technology, Calabar P.M.B. 1123 Calabar, Cross River State, Nigeria Abstract Four Hundred (400) samples of Chrysichthys nigrodigitatus were examined for Henneguya chrysichthys using methods described for gill examination, egg separation and histopathology. Monthly prevalence ranged from 5(14.7%) to 17(51.5%). Highest monthly parasite intensity (5 parasites /kg) was recorded in the month of June and July while highest mean condition factor (0.9900 kg/cm3) was observed in the month of July. 88 (22.0%) and 47 (11.8%) prevalence were recorded for wet and dry seasons respectively. More females (17.3 %) hand infection than males (16.5 %). Infection was highest in 41-50cm, 61cm-70cm and 61cm-70cm in the low moderate and high infection categories. Eighty (20.0%) of 238 (59.5 %) females examined were gravid. 57 (14.3%) of gravid females examined were infected. Absolute 231 Copyright © IAARR 2012: www.afrrevjo.net/stch AFRREV STECH, Vol. 1 (3) August-December, 2012 fecundity range of 3,865 eggs to 28,675 eggs and 3,601 eggs to 24,699 eggs and relative fecundity of 366 and 251 were recorded for uninfected and infected fish respectively. Oocyte diameter varied between 1.0mm and 3.6mm and 0.3mm and 1.8mm for uninfected and infected gravid females. -

Worms, Germs, and Other Symbionts from the Northern Gulf of Mexico CRCDU7M COPY Sea Grant Depositor
h ' '' f MASGC-B-78-001 c. 3 A MARINE MALADIES? Worms, Germs, and Other Symbionts From the Northern Gulf of Mexico CRCDU7M COPY Sea Grant Depositor NATIONAL SEA GRANT DEPOSITORY \ PELL LIBRARY BUILDING URI NA8RAGANSETT BAY CAMPUS % NARRAGANSETT. Rl 02882 Robin M. Overstreet r ii MISSISSIPPI—ALABAMA SEA GRANT CONSORTIUM MASGP—78—021 MARINE MALADIES? Worms, Germs, and Other Symbionts From the Northern Gulf of Mexico by Robin M. Overstreet Gulf Coast Research Laboratory Ocean Springs, Mississippi 39564 This study was conducted in cooperation with the U.S. Department of Commerce, NOAA, Office of Sea Grant, under Grant No. 04-7-158-44017 and National Marine Fisheries Service, under PL 88-309, Project No. 2-262-R. TheMississippi-AlabamaSea Grant Consortium furnish ed all of the publication costs. The U.S. Government is authorized to produceand distribute reprints for governmental purposes notwithstanding any copyright notation that may appear hereon. Copyright© 1978by Mississippi-Alabama Sea Gram Consortium and R.M. Overstrect All rights reserved. No pari of this book may be reproduced in any manner without permission from the author. Primed by Blossman Printing, Inc.. Ocean Springs, Mississippi CONTENTS PREFACE 1 INTRODUCTION TO SYMBIOSIS 2 INVERTEBRATES AS HOSTS 5 THE AMERICAN OYSTER 5 Public Health Aspects 6 Dcrmo 7 Other Symbionts and Diseases 8 Shell-Burrowing Symbionts II Fouling Organisms and Predators 13 THE BLUE CRAB 15 Protozoans and Microbes 15 Mclazoans and their I lypeiparasites 18 Misiellaneous Microbes and Protozoans 25 PENAEID -

Freshwater and Marine Prevalence of Nanophyetus Infections in Puget Sound
Freshwater and Marine Prevalence of Nanophyetus infections in Puget Sound. Martin Chen1,Robert Conrad1,Bruce Stewart1, Paul Hershberger2 and Kevin Snekvik3 1Northwest Indian Fisheries Commission, OlympiaFish Health Assessment of outmigrating steelhead 2USGS‐Western Fisheries Research Center, Marrowstone Island Marine Field Station, Nordland 3Washington Animal Disease Diagnostic Laboratory, Washington State University, Pullman Nanophyetus salmincola lifecycle The first intermediate host is a snail Juga plicifera Juga silicula (Juga formerly known as Oxytrema) Salmon Poisoning Disease of dogs is caused by the bacterium Neorickettsia helminthoeca carried by Nanophyetus salmincola Remove posterior kidney and count numbers of metacercaria. (59) Kidney samples were placed in a sample bag, compressed between two glass plates and examined at 15X. Study Questions: 1. Is there a correlation between the lower return of steelhead to South Sound Rivers and the presence and intensity of N. salmincola infection? 2. Does the intensity of infection as outmigration proceeds suggest a causal effect between N. salmincola and poor marine survival? Sampling Design 5 Puget Sound watersheds Hatcheries Traps Lower River / Estuary 3 Offshore Areas • Whidbey Basin • Green / Duwamish • Nisqually % Prevalence of Nanophyetus salmincola, other parasites and organ pathology in steelhead smolts from four Puget Sound river basins in 2014. (n)= sample size Site N. Kidney Sanguinicola Gill Heart salmincola Myxosporean Pathology Pathology Skagit R. 5 (21) 40 (5) 0 (5) 0 (5) 0 (5) Whidbey 7 (42) 36 (31) 3 (31) 0 (31) 3 (31) Offshore Snohomish R. 0 (7) 20 (5) 0 (5) 0 (5) 0 (5) Green R. 73 (112) 12 (89) 0 (89) 28 (89) 45 (89) South‐Central 94 (15) 36 (14) 14 (14) 7 (14) 29 (14) Offshore Nisqually R. -

D070p001.Pdf
DISEASES OF AQUATIC ORGANISMS Vol. 70: 1–36, 2006 Published June 12 Dis Aquat Org OPENPEN ACCESSCCESS FEATURE ARTICLE: REVIEW Guide to the identification of fish protozoan and metazoan parasites in stained tissue sections D. W. Bruno1,*, B. Nowak2, D. G. Elliott3 1FRS Marine Laboratory, PO Box 101, 375 Victoria Road, Aberdeen AB11 9DB, UK 2School of Aquaculture, Tasmanian Aquaculture and Fisheries Institute, CRC Aquafin, University of Tasmania, Locked Bag 1370, Launceston, Tasmania 7250, Australia 3Western Fisheries Research Center, US Geological Survey/Biological Resources Discipline, 6505 N.E. 65th Street, Seattle, Washington 98115, USA ABSTRACT: The identification of protozoan and metazoan parasites is traditionally carried out using a series of classical keys based upon the morphology of the whole organism. However, in stained tis- sue sections prepared for light microscopy, taxonomic features will be missing, thus making parasite identification difficult. This work highlights the characteristic features of representative parasites in tissue sections to aid identification. The parasite examples discussed are derived from species af- fecting finfish, and predominantly include parasites associated with disease or those commonly observed as incidental findings in disease diagnostic cases. Emphasis is on protozoan and small metazoan parasites (such as Myxosporidia) because these are the organisms most likely to be missed or mis-diagnosed during gross examination. Figures are presented in colour to assist biologists and veterinarians who are required to assess host/parasite interactions by light microscopy. KEY WORDS: Identification · Light microscopy · Metazoa · Protozoa · Staining · Tissue sections Resale or republication not permitted without written consent of the publisher INTRODUCTION identifying the type of epithelial cells that compose the intestine.