Mechanics of Cuticular Elastic Energy Storage in Leg Joints Lacking Extensor Muscles in Arachnids Andrew T
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Order Ephemeroptera
Glossary 1. Abdomen: the third main division of the body; behind the head and thorax 2. Accessory flagellum: a small fingerlike projection or sub-antenna of the antenna, especially of amphipods 3. Anterior: in front; before 4. Apical: near or pertaining to the end of any structure, part of the structure that is farthest from the body; distal 5. Apicolateral: located apical and to the side 6. Basal: pertaining to the end of any structure that is nearest to the body; proximal 7. Bilobed: divided into two rounded parts (lobes) 8. Calcareous: resembling chalk or bone in texture; containing calcium 9. Carapace: the hardened part of some arthropods that spreads like a shield over several segments of the head and thorax 10. Carinae: elevated ridges or keels, often on a shell or exoskeleton 11. Caudal filament: threadlike projection at the end of the abdomen; like a tail 12. Cercus (pl. cerci): a paired appendage of the last abdominal segment 13. Concentric: a growth pattern on the opercula of some gastropods, marked by a series of circles that lie entirely within each other; compare multi-spiral and pauci-spiral 14. Corneus: resembling horn in texture, slightly hardened but still pliable 15. Coxa: the basal segment of an arthropod leg 16. Creeping welt: a slightly raised, often darkened structure on dipteran larvae 17. Crochet: a small hook-like organ 18. Cupule: a cup shaped organ, as on the antennae of some beetles (Coleoptera) 19. Detritus: disintegrated or broken up mineral or organic material 20. Dextral: the curvature of a gastropod shell where the opening is visible on the right when the spire is pointed up 21. -

Gene Duplication and the Origins Of
Rivera et al. BMC Evolutionary Biology 2010, 10:123 http://www.biomedcentral.com/1471-2148/10/123 Daphnia Genomics Consortium RESEARCH ARTICLE Open Access Gene duplication and the origins of morphological complexity in pancrustacean eyes, a genomic approach Ajna S Rivera1, M Sabrina Pankey1, David C Plachetzki1, Carlos Villacorta1, Anna E Syme1, Jeanne M Serb1,3, Angela R Omilian2, Todd H Oakley1* Abstract Background: Duplication and divergence of genes and genetic networks is hypothesized to be a major driver of the evolution of complexity and novel features. Here, we examine the history of genes and genetic networks in the context of eye evolution by using new approaches to understand patterns of gene duplication during the evolution of metazoan genomes. We hypothesize that 1) genes involved in eye development and phototransduction have duplicated and are retained at higher rates in animal clades that possess more distinct types of optical design; and 2) genes with functional relationships were duplicated and lost together, thereby preserving genetic networks. To test these hypotheses, we examine the rates and patterns of gene duplication and loss evident in 19 metazoan genomes, including that of Daphnia pulex - the first completely sequenced crustacean genome. This is of particular interest because the pancrustaceans (hexapods+crustaceans) have more optical designs than any other major clade of animals, allowing us to test specifically whether the high amount of disparity in pancrustacean eyes is correlated with a higher rate of duplication and retention of vision genes. Results: Using protein predictions from 19 metazoan whole-genome projects, we found all members of 23 gene families known to be involved in eye development or phototransduction and deduced their phylogenetic relationships. -

Target-Specificity in Scorpions
Toxins 2017, 9, 312, doi: 10.3390/toxins9100312 S1 of S3 Supplementary Materials: Target‐specificity in scorpions; Comparing lethality of scorpion venoms across arthropods and vertebrates Arie van der Meijden, Bjørn Koch, Tom van der Valk, Leidy J. Vargas‐Muñoz and Sebastian Estrada‐Gómez Table S1. Table with GenBank CO1 accession numbers. Voucher numbers refer to the specimens in the collection of CIBIO/InBio at the University of Porto. Species Voucher Accession number Androctonus australis Sc904 gi379647585 Leiurus quinquestriatus Sc1062 gi379647601 Buthus ibericus Sc112 gi290918312 Grosphus flavopiceus Sc1085 gi379647593 Centruroides gracilis gi71743793 Heterometrus laoticus Sc1084 gi555299473 Pandinus imperator Sc1050 gi379647587 Hadrurus arizonensis Sc1042 gi379647597 Iurus kraepelini Sc866 gi379647589 Table S2. Mean dry venom compound per individual. Several milkings were made per (sub)adult specimen in some cases. Species n Dry venom (mg) mg/milking Androctonus australis 21 23.5 1.12 Leiurus quinquestriatus 51 25.1 0.49 Buthus ibericus 47 41.6 0.89 Centruroides gracilis 42 22.5 0.54 Babycurus jacksoni 38 53.9 1.42 Grosphus grandidieri 19 103.9 5.47 Hadrurus arizonensis 9 75.3 8.37 Iurus sp.* 12 35.2 2.93 Heterometrus laoticus 21 119 5.67 Pandinus imperator 15 72.7 4.85 *2 I. dufoureius, 6 I. kraepelini, 4 I. sp. Toxins 2017, 9, 312, doi: 10.3390/toxins9100312 S2 of S3 Table S3. Toxicological analysis of the venom of Grosphus grandidieri. “Toxic” means that the mice showed symptoms such as: pain, piloerection, excitability, salivation, lacrimation, dyspnea, diarrhea, temporary paralysis, but recovered within 20 h. “Lethal” means that the mice showed some or all the symptoms of intoxication and died within 20 h after injection. -

The Evolution and Development of Arthropod Appendages
Evolving Form and Function: Fossils and Development Proceedings of a symposium honoring Adolf Seilacher for his contributions to paleontology, in celebration of his 80th birthday Derek E. G. Briggs, Editor April 1– 2, 2005 New Haven, Connecticut A Special Publication of the Peabody Museum of Natural History Yale University New Haven, Connecticut, U.S.A. December 2005 Evolving Form and Function: Fossils and Development Proceedings of a symposium honoring Adolf Seilacher for his contributions to paleontology, in celebration of his 80th birthday A Special Publication of the Peabody Museum of Natural History, Yale University Derek E.G. Briggs, Editor These papers are the proceedings of Evolving Form and Function: Fossils and Development, a symposium held on April 1–2, 2005, at Yale University. Yale Peabody Museum Publications Jacques Gauthier, Curatorial Editor-in-Chief Lawrence F. Gall, Executive Editor Rosemary Volpe, Publications Editor Joyce Gherlone, Publications Assistant Design by Rosemary Volpe • Index by Aardvark Indexing Cover: Fossil specimen of Scyphocrinites sp., Upper Silurian, Morocco (YPM 202267). Purchased for the Yale Peabody Museum by Dr. Seilacher. Photograph by Jerry Domian. © 2005 Peabody Museum of Natural History, Yale University. All rights reserved. Frontispiece: Photograph of Dr. Adolf Seilacher by Wolfgang Gerber. Used with permission. All rights reserved. In addition to occasional Special Publications, the Yale Peabody Museum publishes the Bulletin of the Peabody Museum of Natural History, Postilla and the Yale University Publications in Anthropology. A com- plete list of titles, along with submission guidelines for contributors, can be obtained from the Yale Peabody Museum website or requested from the Publications Office at the address below. -
Scorpion Tufts.Pdf
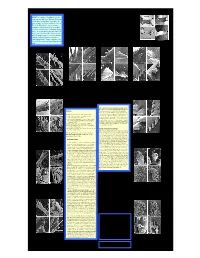
ABSTRACT. Five scorpion genera of superfamily Iuroidea exhibit Tarsal Spinule Clusters and Evolution of the ancient disjunct ranges (South America, North America, Mediterranean), and are an important object in the study of scorpion phylogeny. They have an exceptional variety of tarsal leg setation/spination (Soleglad & Fet 2003). New SEM data from all five genera and two families: Superfamily Iuroidea (Scorpiones) Caraboctonidae (Caraboctonus, Hadruroides, Hadrurus) and Iuridae (Iurus, Calchas) are characterized in detail. We demonstrate two major patterns: (1) an irregular median row of grouped spinule clusters, found in juvenile to subadult but reduced in adult (Calchas); or (2) a median 1 2 3 4 row of highly concentrated spinule clusters. Pattern (2) is either forming Victor Fet , Michael E. Soleglad , David P.A. Neff & Iasmi Stathi “spinule tufts” (Caraboctonus, Hadruroides, Iurus), or individual 1Department of Biological Sciences, and 3Department of Chemistry, Marshall University, Huntington, WV, USA; “spinule-looking” protuberances (Hadrurus). We suggest that the latter Hadrurus obscurus are a derived feature as a result of fusion of separate spinules into a solid 2Borrego Springs, CA, USA; 3Department of Zoology, University of Crete, Irakleio, Crete, Greece structure. General appearance of tarsal armament in Iuroidea Figs. 17-20. Lateral-ventral view of leg III tarsus of Hadrurus a. arizonensis. 17. Figs. 21-24. Lateral-ventral view of leg III (leg IV in Fig. 24) tarsus of Mexican Figs. 25-28. Lateral-ventral view of leg III tarsus of Baja California Hadrurus species. Closeup of fused spinule cluster of adult (carapace length = xx.x mm). Borrego Hadrurus species. 21. Closeup of fused spinule cluster of adult H. -

Soleglad, Fet & Lowe: Hadrurus “Spadix” Subgroup 17
Soleglad, Fet & Lowe: Hadrurus “spadix” Subgroup 17 Figures 31–33 Comparisons of Hadrurus obscurus and H. spadix, metasomal segments II–III, ventral view, showing diagnostic setation located between the ventromedian (VM) carinae. 31. H. obscurus, male (pale phenotype, segment II length = 8.44 mm, segment III length = 9.26 mm), Bird Spring Canyon Road, Kern Co., California, USA. 32. H. obscurus, female (dark phenotype, segment II length = 5.02 mm, segment III length = 5.70 mm), Bird Spring Canyon Road, Kern Co., California, USA. 33. H. spadix, female (segment II length = 7.87 mm, segment III length = 8.36 mm), Apex Mine in Curly Hollow Wash, Washington Co., Utah, USA. 19). However, to support our suspicion, we have ex- As stated above the positions and numbers of chelal amined two H. obscurus specimens collected from the internal trichobothria are essentially identical in Ha- same locality where one has a carapace pattern of H. drurus obscurus and H. spadix, while both numbers and spadix and the other a pattern typical of H. obscurus (see positions differ in H. anzaborrego (see Fig. 19). H. Fig. 20 for color closeup images of these carapaces, and anzaborrego has three internal accessory trichobothria Figs. 9–10 for overall comparison with “arizonensis” whereas the other two species have two. Statistics group species). Based on these data, we suggest here that involving over 250 samples show that these numerical these carapacial pattern differences are analogous to differences are observed in over 87 % of the specimens those exhibited by the dark and pale phenotypes of H. examined (Fig. -

Euscorpius. 2009
Euscorpius Occasional Publications in Scorpiology Courtship and Mating in Heterometrus petersii (Thorell, 1876) (Scorpiones: Scorpionidae) Guo-Bin Jiao & Ming-Sheng Zhu June 2009 – No. 84 Euscorpius Occasional Publications in Scorpiology EDITOR: Victor Fet, Marshall University, ‘[email protected]’ ASSOCIATE EDITOR: Michael E. Soleglad, ‘[email protected]’ Euscorpius is the first research publication completely devoted to scorpions (Arachnida: Scorpiones). Euscorpius takes advantage of the rapidly evolving medium of quick online publication, at the same time maintaining high research standards for the burgeoning field of scorpion science (scorpiology). Euscorpius is an expedient and viable medium for the publication of serious papers in scorpiology, including (but not limited to): systematics, evolution, ecology, biogeography, and general biology of scorpions. Review papers, descriptions of new taxa, faunistic surveys, lists of museum collections, and book reviews are welcome. Derivatio Nominis The name Euscorpius Thorell, 1876 refers to the most common genus of scorpions in the Mediterranean region and southern Europe (family Euscorpiidae). Euscorpius is located on Website ‘http://www.science.marshall.edu/fet/euscorpius/’ at Marshall University, Huntington, WV 25755-2510, USA. The International Code of Zoological Nomenclature (ICZN, 4th Edition, 1999) does not accept online texts as published work (Article 9.8); however, it accepts CD-ROM publications (Article 8). Euscorpius is produced in two identical versions: online (ISSN 1536-9307) -

Defensive Behavior Is Associated with Morphology and Performance in Scorpions
Choose Your Weapon: Defensive Behavior Is Associated with Morphology and Performance in Scorpions Arie van der Meijden1*, Pedro Lobo Coelho1, Pedro Sousa1, Anthony Herrel2 1 CIBIO, Centro de Investigac¸a˜o em Biodiversidade e Recursos Gene´ticos, Campus Agra´rio de Vaira˜o, Vaira˜o, Portugal, 2 UMR 7179, Muse´um National d9Histoire Naturelle, De´partement d9Ecologie et de Gestion de la Biodiversite´, Paris, France Abstract Morphology can be adaptive through its effect on performance of an organism. The effect of performance may, however, be modulated by behavior; an organism may choose a behavioral option that does not fully utilize its maximum performance. Behavior may therefore be decoupled from morphology and performance. To gain insight into the relationships between these levels of organization, we combined morphological data on defensive structures with measures of defensive performance, and their utilization in defensive behavior. Scorpion species show significant variation in the morphology and performance of their main defensive structures; their chelae (pincers) and the metasoma (‘‘tail’’) carrying the stinger. Our data show that size-corrected pinch force varies to almost two orders of magnitude among species, and is correlated with chela morphology. Chela and metasoma morphology are also correlated to the LD50 of the venom, corroborating the anecdotal rule that dangerously venomous scorpions can be recognized by their chelae and metasoma. Analyses of phylogenetic independent contrasts show that correlations between several aspects of chela and metasoma morphology, performance and behavior are present. These correlations suggest co-evolution of behavior with morphology and performance. Path analysis found a performance variable (pinch force) to partially mediate the relationship between morphology (chela aspect ratio) and behavior (defensive stinger usage). -

Scorpion Phylogeography in the North American Aridlands
UNLV Theses, Dissertations, Professional Papers, and Capstones 8-1-2012 Scorpion Phylogeography in the North American Aridlands Matthew Ryan Graham University of Nevada, Las Vegas Follow this and additional works at: https://digitalscholarship.unlv.edu/thesesdissertations Part of the Biology Commons, Desert Ecology Commons, and the Population Biology Commons Repository Citation Graham, Matthew Ryan, "Scorpion Phylogeography in the North American Aridlands" (2012). UNLV Theses, Dissertations, Professional Papers, and Capstones. 1668. http://dx.doi.org/10.34917/4332649 This Dissertation is protected by copyright and/or related rights. It has been brought to you by Digital Scholarship@UNLV with permission from the rights-holder(s). You are free to use this Dissertation in any way that is permitted by the copyright and related rights legislation that applies to your use. For other uses you need to obtain permission from the rights-holder(s) directly, unless additional rights are indicated by a Creative Commons license in the record and/or on the work itself. This Dissertation has been accepted for inclusion in UNLV Theses, Dissertations, Professional Papers, and Capstones by an authorized administrator of Digital Scholarship@UNLV. For more information, please contact [email protected]. SCORPION PHYLOGEOGRAPHY IN THE NORTH AMERICAN ARIDLANDS by Matthew Ryan Graham Bachelor of Science Marshall University 2004 Master of Science Marshall University 2007 A dissertation submitted in partial fulfillment of the requirements for the Doctor of Philosophy in Biological Sciences School of Life Sciences College of Sciences The Graduate College University of Nevada, Las Vegas August 2012 Copyright by Matthew R. Graham, 2012 All Rights Reserved THE GRADUATE COLLEGE We recommend the thesis prepared under our supervision by Matthew R. -

Exploring the Origin of Insect Wings from an Evo-Devo Perspective
Available online at www.sciencedirect.com ScienceDirect Exploring the origin of insect wings from an evo-devo perspective Courtney M Clark-Hachtel and Yoshinori Tomoyasu Although insect wings are often used as an example of once (i.e. are monophyletic) sometime during the Upper morphological novelty, the origin of insect wings remains a Devonian or Lower Carboniferous (370-330 MYA) [3,5,9]. mystery and is regarded as a major conundrum in biology. Over By the early Permian (300 MYA), winged insects had a century of debates and observations have culminated in two diversified into at least 10 orders [4]. Therefore, there is prominent hypotheses on the origin of insect wings: the tergal quite a large gap in the fossil record between apterygote hypothesis and the pleural hypothesis. However, despite and diverged pterygote lineages, which has resulted in a accumulating efforts to unveil the origin of insect wings, neither long-running debate over where insect wings have come hypothesis has been able to surpass the other. Recent from and how they have evolved. investigations using the evolutionary developmental biology (evo-devo) approach have started shedding new light on this Two proposed wing origins century-long debate. Here, we review these evo-devo studies The insect wing origin debate can be broken into two and discuss how their findings may support a dual origin of main groups of thought; wings evolved from the tergum of insect wings, which could unify the two major hypotheses. ancestral insects or wings evolved from pleuron-associat- Address ed structures (Figure 1. See Box 1 for insect anatomy) Miami University, Pearson Hall, 700E High Street, Oxford, OH 45056, [10 ]. -

AC07942458.Pdf
From the Research Institute of Wildlife Ecology University of Veterinary Medicine Vienna (Department head: O. Univ. Prof. Dr. rer. net. Walter Arnold) THE HEMOLYMPH COMPOSITION OF THE'AFRICAN EMPEROR SCORPION {PANDINUSIMPERATOR) & SUGGESTIONS FOR THE USE OF PARENTERAL FLUIDS IN DEHYDRATED AFRICAN EMPEROR SCORPIONS MASTER THESIS by Melinda de Mul Vienna, August 2009 1st Reviewer: Univ.Prof. Dr.med.vet. Tzt. Christian Walzer 2"d Reviewer: Ao.Univ.Prof. Dr.rer.nat. Thomas Ruf 3^^ Reviewer: Ao.Univ.Prof. Dr.med.vet. Tzt. Franz Schwarzenberger Contents 1 Introduction -11 Anatomy and Physiology -12 Morphology -12 Integumentum -12 Alimentary tract -13 Respiratory system -14 Cardiovascular system -14 Hemolymph and hemocytes -15 Fluid ba\aLX\(^ oi Pandinus impemtor -15 Water-conserving mechanisms -16 Water-regaining mechanisms -16 Fluid deficit In scorpions -17 Causes -17 Symptoms -17 Diagnostics -17 Treatment -18 2 Materials & Methods -19 Animals -19 Housing -19 Feeding - 20 Scorpion Immobilisation - 20 Hemolymph withdrawal - 21 Hemolymph analysis - 21 Electrolytes and Osmolality - 21 Metallic elements - 22 Statistics -22 3 Results -23 Differences between sampling times - 23 Correlations - 25 Distribution of the data - 25 Contents Potassium - 25 Magnesium -26 4 Discussion & Conclusion - 27 Hemolymph composition and osmolallty - 27 Interspecific differences - 28 PQTf\is\ox\f{y]MiS for Pandinus Imperator - 31 Conclusion - 34 5 Summary - 37 6 Zusammenfassung - 39 7 Samenvatting - 41 8 Acknowledgements - 43 9 References - 45 Index of figures Figure 1.1. Body structure of an adult Pandinus Imperator, dorsal view. * paired median eyes -12- Figure 1.2. Body structure of an adult Pandinus Imperator, vetral view: p, pectines; s, spiracles -13- Figure 1.3. -

How to Align Arthropod Leg Segments
bioRxiv preprint doi: https://doi.org/10.1101/2021.01.20.427514; this version posted January 21, 2021. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY 4.0 International license. How to align arthropod leg segments Authors: Heather S. Bruce1,2* 5 Affiliations: 1. University of California Berkeley, Berkeley, CA. 2. Marine Biological Laboratory, Woods Hole, MA. *Correspondence to: [email protected] 10 Abstract How to align leg segments between the four groups of arthropods (insects, crustaceans, myriapods, and chelicerates) has tantalized researchers for over a century. By comparing the loss-of-function phenotypes of leg patterning genes in diverged arthropod taxa, including a crustacean, insects, and arachnids, arthropod legs can be aligned in a one-to-one fashion. By 15 comparing the expression of pannier and aurucan, the proximal leg segments can be aligned. A model is proposed wherein insects and myriapods incorporated the proximal leg region into the body wall, which moved an ancestral exite (for example, a gill) on the proximal leg into the body wall, where it invaginated independently in each lineage to form tracheae. For chelicerates with seven leg segments, it appears that one proximal leg segment was incorporated into the body 20 wall. According to this model, the chelicerate exopod and the crustacean exopod emerge from different leg segments, and are therefore proposed to have arisen independently. A framework for how to align arthropod appendages now opens up a powerful system for studying the origins of novel structures, the plasticity of developmental fields across vast phylogenetic distances, and the convergent evolution of shared ancestral developmental fields.