Life History of the Bog Buck Moth (Saturniidae: Hemileuca) in New York State
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Bogbean Buckmoth (Hemileuca Sp.) in Canada
PROPOSED Species at Risk Act Recovery Strategy Series Adopted under Section 44 of SARA Recovery Strategy for the Bogbean Buckmoth (Hemileuca sp.) in Canada Bogbean Buckmoth 2015 1 Recommended citation: Environment Canada. 2015. Recovery Strategy for the Bogbean Buckmoth (Hemileuca sp.) in Canada [Proposed]. Species at Risk Act Recovery Strategy Series. Environment Canada, Ottawa. 18 pp. + Appendix. For copies of the recovery strategy, or for additional information on species at risk, including COSEWIC Status Reports, residence descriptions, action plans, and other related recovery documents, please visit the Species at Risk Public Registry1. Cover illustration: © Don Cuddy Également disponible en français sous le titre « Programme de rétablissement de l’hémileucin du ményanthe (Hemileuca sp.) au Canada [Proposition] » © Her Majesty the Queen in Right of Canada, represented by the Minister of the Environment, 2015. All rights reserved. ISBN Catalogue no. Content (excluding the illustrations) may be used without permission, with appropriate credit to the source. 1 http://sararegistry.gc.ca/default.asp?lang=En&n=24F7211B-1 RECOVERY STRATEGY FOR THE BOGBEAN BUCKMOTH (Hemileuca sp.) IN CANADA 2015 Under the Accord for the Protection of Species at Risk (1996), the federal, provincial, and territorial governments agreed to work together on legislation, programs, and policies to protect wildlife species at risk throughout Canada. In the spirit of cooperation of the Accord, the Government of Ontario has given permission to the Government of Canada to adopt the Recovery Strategy for Bogbean Buckmoth (Hemileuca sp.) in Ontario (Part 2) under Section 44 of the Species at Risk Act. Environment Canada has included an addition (Part 1) which completes the SARA requirements for this recovery strategy. -
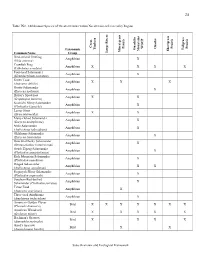
State Overview and Ecological Framework Table IN2. Oklahoma's
24 Table IN2. Oklahoma's Species of Greatest Conservation Need Cross-referenced by Region grass - Cross Prairie Prairie Prairie WGCP Ozarks Timbers Tallgrass Taxonomic Ouachita Mountains Shortgrass Mixed Common Name Group Large Rivers Bird-voiced Treefrog Amphibian X (Hyla avivoca) Crawfish Frog Amphibian X X X X (Lithobates areolata) Four-toed Salamander Amphibian X (Hemidactylium scutatum) Green Toad Amphibian X X X (Anaxyrus debilis) Grotto Salamander Amphibian X (Eurycea spelaeus) Hurter's Spadefoot Amphibian X X (Scaphiopus hurterii) Kiamichi Slimy Salamander Amphibian X (Plethodon kiamichi) Lesser Siren Amphibian X X (Siren intermedia) Many-ribbed Salamander Amphibian X (Eurycea multiplicata) Mole Salamander Amphibian X (Ambystoma talpoideum) Oklahoma Salamander Amphibian X (Eurycea tynerensis) Ouachita Dusky Salamander Amphibian X (Desmognathus brimleyorum) Ozark Zigzag Salamander Amphibian X (Plethodon angusticlavius) Rich Mountain Salamander Amphibian X (Plethodon ouachitae) Ringed Salamander Amphibian X X (Ambystoma annulatum) Sequoyah Slimy Salamander Amphibian X (Plethodon sequoyah) Southern Red-backed Amphibian X Salamander (Plethodon serratus) Texas Toad Amphibian X (Anaxyrus speciosus) Three-toed Amphiuma Amphibian X (Amphiuma tridactylum) American Golden Plover Bird X X X X X X X (Pluvialis dominica) American Woodcock Bird X X X X X (Scolopax minor) Bachman's Sparrow Bird X X X X (Aimophila aestivalis) Baird's Sparrow Bird X X (Ammodramus bairdii) State Overview and Ecological Framework 25 grass - ntains Cross Large Rivers -

The Taxonomic Report of the INTERNATIONAL LEPIDOPTERA SURVEY
Volume 8 Number 5 1 April, 2020 The Taxonomic Report OF THE INTERNATIONAL LEPIDOPTERA SURVEY ISSN 2643-4776 (print) / ISSN 2643-4806 (online) A phenotypic comparison of regional populations of Hemileuca maia (Drury, 1773) with designations of new subspecies (Bombycoidea, Saturniidae, Hemileucinae). Harry Pavulaan 606 Hunton Place NE Leesburg, VA. 20176 [email protected] ABSTRACT. Following refinement of the type locality of Hemileuca maia to the Long Island Pine Barrens of New York State by the author (Pavulaan, 2020), an evaluation of phenotypic characters of regional populations of H. maia is presented. The Long Island population is the nominotypical subspecies. Populations in southeastern coastal New England and offshore islands are presently considered nominotypical maia. However, several continental inland populations show evidence of subspecific variation. Four new subspecies are designated. Detailed phenotypic information of other interior regions is lacking. Additional key words: Pitch Pine Barrens, Scrub Oak Plains, isolate, Menyanthes trifoliata. ZooBank registration: urn:1sid:zoobank.org:pub:3595D21C-4FDE-4336-A588-4E68195E1118 INTRODUCTION The Buckmoths of North America are a bewildering blend of intergrading phenotypes that have been the subject of numerous studies (Ferguson, 1971; Tuskeset al., 1996; Rubinoffet al., 2017; Dupuiset al., 2018). Results of these studies are inconclusive over where to draw taxonomc limits. Michener (1952) proposed a subdivision of genus Hemileuca into four subgenera: Hemileuca (Walker, 1855), Pseudohazis (Grote & Robinson, 1866), Euleucophaeus (Packard, 1872) and Argyrauges (Grote, 1882). Nestled within subgenus Hemileuca is the Hemileuca maia complex, presently considered to be a closely- related group of species and unnamed populations of species H. maia. This group is characterized by variation in ground color (gray to black), bold median bands (white to yellow), and scale translucence. -

Moths of Ohio Guide
MOTHS OF OHIO field guide DIVISION OF WILDLIFE This booklet is produced by the ODNR Division of Wildlife as a free publication. This booklet is not for resale. Any unauthorized INTRODUCTION reproduction is prohibited. All images within this booklet are copyrighted by the Division of Wildlife and it’s contributing artists and photographers. For additional information, please call 1-800-WILDLIFE. Text by: David J. Horn Ph.D Moths are one of the most diverse and plentiful HOW TO USE THIS GUIDE groups of insects in Ohio, and the world. An es- Scientific Name timated 160,000 species have thus far been cata- Common Name Group and Family Description: Featured Species logued worldwide, and about 13,000 species have Secondary images 1 Primary Image been found in North America north of Mexico. Secondary images 2 Occurrence We do not yet have a clear picture of the total Size: when at rest number of moth species in Ohio, as new species Visual Index Ohio Distribution are still added annually, but the number of species Current Page Description: Habitat & Host Plant is certainly over 3,000. Although not as popular Credit & Copyright as butterflies, moths are far more numerous than their better known kin. There is at least twenty Compared to many groups of animals, our knowledge of moth distribution is very times the number of species of moths in Ohio as incomplete. Many areas of the state have not been thoroughly surveyed and in some there are butterflies. counties hardly any species have been documented. Accordingly, the distribution maps in this booklet have three levels of shading: 1. -

Io Moth (Automeris Io)
CLOSE ENCOUNTERS WITH THE ENVIRONMENT What’s Eating You? Io Moth (Automeris io) Eric W. Hossler, MD; Dirk M. Elston, MD; David L. Wagner, PhD f the 7 species of Automeris moths (order, About 10 days after being deposited, minute 2- to Lepidoptera; family, Saturniidae) found in the 3-mm larvae emerge from the ova and feed gregari- O United States, Automeris io often is the most ously upon their host plant. Favorite foods of the Io common and familiar. Its range extends as far north caterpillars include azaleas, birch, blackberry, cherry, as Quebec, Ontario, and southern Manitoba, Canada; clover, cotton, currant, elm, hackberry, hibiscus, west to Utah, Colorado, Nebraska, and Texas; and mesquite, oak, pear, poplar, redbud, rose, sassafras, south to Florida, eastern Mexico, and Costa Rica.1-3 and willow.1,2,4 In addition, larvae frequently feed on Across much of its range, the moths and their lar- grasses such as corn or Bermuda grass.2,4 Io moths are vae are among the most common giant silk moths around in deciduous woodlands, forests, and fields; encountered by the public. In Louisiana, the closely along power line rights-of-way; and in orchards, related Automeris louisiana largely replaces the Io parks, and suburban yards.2,5 moth in coastal areas.2 The sexually dimorphic adults have a wingspan The Io moth has 4 life stages: egg, larva (or cat- of 2.0 to 3.5 in and are easily recognized by the pres- erpillar), pupa, and adult. Eclosion of Io moths from ence of prominent black to blue eyespots with white cocoons occurs during late morning or early evening. -

App B APB Pine Barrens Viability Assessment
Appendix B. Albany Pine Bush Pine Barrens Viability Assessment Albany Pine Bush Pine Barrens Viability Assessment Quantifiable indicators of pine barrens size and extent, fragmentation and edge effects, prescribed fire regime, and biotic patterns Prepared for: Albany Pine Bush Preserve Commission Prepared by: Jason Bried Preserve Ecologist And Neil Gifford Conservation Director November 2008 Albany Pine Bush Pine Barrens Viability Assessment November 2008 Table of Contents Acknowledgements ........................................................................................................... 3 I. INTRODUCTION ......................................................................................................... 4 Planning & Conservation Target .......................................................................... 4 Albany Pine Bush .................................................................................................. 6 Coarse vs. Fine Filter Monitoring ........................................................................ 8 Assessment Framework ......................................................................................... 8 Literature Cited................................................................................................................. 12 II. Size & Extent .............................................................................................................. 17 Habitat amount .................................................................................................... 18 -

Appendix M-7 Buck Moth Study
The Hills at Southampton MUPDD Application Draft EIS Appendix M-7 Buck Moth Study Hugh McGuinness, PhD. Assessment of Coastal Buck Moth (Hemileuca maia) Populations at the Spinney Road Site, East Quogue, New York By Hugh McGuinness, Ph.D.* The Coastal Buck Moth (Hemileuca maia) is a charismatic, day‐flying member of the Giant Silk Moth family (Saturniidae). It belongs to a complex of sibling species that feed on oaks (Quercus spp.) and range throughout the eastern United States. On Long Island, the Buck Moth is restricted to stands of Scrub Oak (Quercus ilicifolia), a shrubby oak species that occurs within pine‐barrens habitats. The Buck Moth appears to favor stands that have not been overtopped by the canopy of pines (mainly Pinus rigida) and other oak species. The Coastal Buck Moth is listed by the New York State Department of Environmental Conservation (NYS DEC) as a rare species of “special concern.” Its state ranking of S1 means that it is “critically imperiled because of rarity…or factors making it especially vulnerable to extinction…in New York State.” The primary reason for its rarity is that the host plant is uncommon and patchy in distribution because it is dependent on disturbance (typically fire) to maintain the open, sandy habitats in which it thrives. In the last 50‐100 years the host plant range in New York has decreased due to fire suppression and to the development of barrens habitats. As a result Buck Moth populations have also decreased. Buck Moths appear to readily respond to newly opened patches of Scrub Oak created by fire. -

2017 Management Plan Update
2017 Management Plan Update For THE ALBANY PINE BUSH PRESERVE March 2017 Globally Rare, Nationally Significant, Locally Distinct 2017 Management Plan Update For The Albany Pine Bush Preserve ________________________________________________________________________ Location of Action: Albany County, City of Albany, Town of Colonie, Town of Guilderland, and Village of Colonie Lead Agency: Albany Pine Bush Preserve Commission 195 New Karner Road Albany, New York 12205 Contact Person: Christopher Hawver, Executive Director Prepared by: Albany Pine Bush Preserve Commission Date: March 16, 2017 The Mission of the Albany Pine Bush Preserve Commission is: TO PROTECT AND MANAGE THE UNIQUE AND ENDANGERED NATURAL COMMUNITIES AND SPECIES OF THE ALBANY PINE BUSH, FOR ECOLOGICAL BENEFITS AND CONTROLLED AND APPROPRIATE PUBLIC RECREATIONAL AND EDUCATIONAL USE. ABOUT THIS MANAGEMENT PLAN UPDATE 1. What is the action? The adoption and implementation of a Management Plan Update for the Albany Pine Bush Preserve. 2. Is the action subject to SEQRA? At its September 15, 2016 meeting the Commission classified the 2017 Draft Management Plan Update for the Albany Pine Bush Preserve as a Type 2 Action consistent with the State Environmental Quality Review Act (SEQRA). 3. Who is proposing to do this? The Albany Pine Bush Preserve Commission. The Commission is comprised of representatives of the Towns of Colonie and Guilderland, the City of Albany, Albany County, The Nature Conservancy, the NYS Department of Environmental Conservation, the NYS Office of Parks, Recreation and Historic Preservation and four private citizens. 4. Why was this report written? The Legislation establishing the Albany Pine Bush Preserve requires that the Preserve Management Plan be reviewed and, if necessary, updated every five years. -

Body Temperature, Behavior, and Growth of Earl Y-Spring Caterpillars (Hemileuca Lucina: Saturniidae)
Journal of the Lepidopterists' Society 44(3). 1990. 143-155 BODY TEMPERATURE, BEHAVIOR, AND GROWTH OF EARL Y-SPRING CATERPILLARS (HEMILEUCA LUCINA: SATURNIIDAE) NANCY E. STAMP Department of Biological Sciences, State University of New York, Binghamton, New York 13901 AND M. DEANE BOWERS University of Colorado Museum and Department of E.P.O. Biology, Campus Box 334, University of Colorado, Boulder, Colorado 80309 ABSTRACT. Buckmoth caterpillars (Hemileuca lucina: Saturniidae) hatch in May, are then exposed to considerable variation in spring thermal conditions, and thus may benefit by basking. We found that larvae exposed to full sunlight reached body temper atures as much as 5°C above ambient temperatures. When sunlight was obscured by clouds, their body temperatures rapidly cooled to that of ambient. However, during partly sunny conditions, larvae in groups were often warmer than solitary larvae. The advantages for buck moth caterpillars in attaining body temperatures warmer than ambient are discussed in terms of their food plant and predators. Additional key words: basking, gregarious, predators, Spiraea latifolia, Massachu setts. Thermal conditions affect the foraging patterns of and food plant exploitation by herbivorous insects. For example, raising the body tem perature by basking may speed up physiological processes enabling larvae to consume and digest food faster and, thus, develop more quickly (Sherman & Watt 1973, Casey 1976, Grossmueller & Lederhouse 1985). Shortened developmental time for larvae may decrease availability to parasitoids (Porter 1983) and predators (Evans 1982) and maximize the intake of high quality food, which may be available only for a limited time during the growing season (Feeny 1970, Stamp & Bowers 1990a). -
MOTHS of OHIO Field Guide DIVISION of WILDLIFE INTRODUCTION HOW to USE THIS GUIDE Text By: David J
MOTHS OF OHIO field guide DIVISION OF WILDLIFE INTRODUCTION HOW TO USE THIS GUIDE Text by: David J. Horn Ph.D Scientific Name Common Name Moths are one of the most diverse and plentiful Group and Family Description: Featured Species groups of insects in Ohio, and the world. An esti- Secondary images 1 Primary Image mated 160,000 species have thus far been catalogued Secondary images 2 Occurrence worldwide, and about 13,000 species have been Size: when at rest found in North America north of Mexico. We do not Visual Index Ohio Distribution yet have a clear picture of the total number of moth Current Page species in Ohio, as new species are still added annu- Description: Habitat & Host Plant Credit & Copyright ally, but the number of species is certainly over 3,000. Although not as popular as butterflies, moths are far Compared to many groups of animals, our knowledge of moth distribution is very more numerous than their better known kin. There is incomplete. Many areas of the state have not been thoroughly surveyed and in some at least twenty times the number of species of moths counties hardly any species have been documented. Accordingly, the distribution maps in Ohio as there are butterflies. in this booklet have three levels of shading: 1. heavily-shaded means a species record documented by specimen or photograph and confirmed by the Ohio Lepidop- The world of moths is one of extraordinary terists. 2. Intermediate shading indicates that the moth is almost certainly present beauty, fantastic behavior, and outrageous diversity. and could be found at the right season. -

Biogeography of a Disjunct Population of Hemileuca Peigleri (Lepidoptera
Journal of Entomology and Zoology Studies 2016; 4(1): 543-549 E-ISSN: 2320-7078 P-ISSN: 2349-6800 Biogeography of a disjunct population of Hemileuca JEZS 2016; 4(1): 543-549 peigleri (Lepidoptera: Saturniidae) in the coastal © 2016 JEZS Received: 15-12-2015 bend of Texas Accepted: 17-01-2016 Robert J. Nuelle, Jr. AICEZS – Research Associate - Robert J. Nuelle, Jr.; Robert J. Nuelle, III; Dr. Shakhawat Bhuiyan; Sam Houston State Natural Dr. William Godwin History Collections, Curator - East Texas Natural History Collection Abstract DNA barcoding and genitalia morphology are used to confirm the identity of an isolated population of Robert J. Nuelle, III Hemileuca in Calhoun County, Texas. Evidence from mitochondrial DNA, genitalia dissection, and Research Associate - Sam morphological evidence supports a species determination of Hemileuca peigleri Lemaire, 1981 despite Houston State Natural History isolation of the population by host plant and apparent geographic distance from the only other known Collections, Research Associate - populations on the Edwards plateau of Texas. East Texas Natural History Collection Keywords: Hemileuca peigleri, buck moths, Saturniidae, Hemileucinae, Calhoun County, Texas Dr. Shakhawat Bhuiyan Ph.D., – Lead Professor of Introduction Biology and Environmental Moths in the genus Hemileuca are members of the family Saturniidae, first described by Sciences at Jarvis Christian Walker in 1855. “The genus Hemileuca, contains more than 35 species ranging from Canada College to Southern Mexico” (Steele & Peigler, 2015) [1]. Sometimes referred to as “buck moths”, these day flying moths are associated with a wide variety of host plants, among them Quercus, Dr. William Godwin Artemisia, Eriogonum and Mimosa (Stone, 1991) [2]. -

Research Publications
Albany Pine Bush Preserve Commission. 2010. Management Plan and Final Environmental Impact Statement for the Albany Pine Bush Preserve. Albany, New York. Albany Pine Bush Preserve Commission. 2017. Management Plan Update for the Albany Pine Bush Preserve. Albany, New York. Albany Pine Bush Preserve Commission. 1999. West overlook pine bush burn: report on the April 27, 1999 incident, recommendations for updated prescribed burn protocol. Albany, NY. Austin, G. T. 2002. Aspen invasion in the Albany Pine Bush. MS Thesis. State University of New York at Albany, Albany, NY. Barnes, J. K. 2003. Natural History of the Albany Pine Bush: Albany and Schenectady counties, New York. New York State Museum Bulletin no. 502. Albany, New York. Barnett, K., and B. Abbuhl. 2007. Ecology and behavior of the eastern hognose snake in upstate NY. Albany Pine Bush Preserve Commission Internal Report. Beachy, B. 2002. Invading trees and breeding birds in the Albany Pine Bush. MS Thesis. State University of New York at Albany, Albany, NY. Beachy, B. L., and G. R. Robinson. 2008. Divergence in avian communities following woody plant invasions in a pine barrens ecosystem. Natural Areas Journal 28:395-403. Benjamins, M. 2003. Effects of shade on the oviposition preferences of the endangered Karner blue butterfly, Lycaeides melissa samuelis Nabokov. M.S. Thesis. College of Environmental Science and Forestry, State University of New York, Syracuse, NY. Bidwell, B., S. Gebauer-Gifford, A. Rudich, and N.A. Gifford. 1998. Short term effects of fire on wild blue lupine in the Albany Pine Bush Preserve, Albany, NY. Albany Pine Bush Preserve Commission Internal Report.