Buck Moth Hemileuca Maia (Drury)1 Clare Scott and Phillip E
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Biology and Distribution of California Hemileucinae (Saturniidae)
Journal of the Lepidopterists' Society 38(4), 1984,281-309 THE BIOLOGY AND DISTRIBUTION OF CALIFORNIA HEMILEUCINAE (SATURNIIDAE) PAUL M. TUSKES 7900 Cambridge 141G, Houston, Texas 77054 ABSTRACT. The distribution, biology, and larval host plants for the 14 species and subspecies of California Hemileucinae are discussed in detail. In addition, the immature stages of Hemileuca neumogeni and Coloradia velda are described for the first time. The relationships among the Hemileuca are examined with respect to six species groups, based on adult and larval characters, host plant relationships and pheromone interactions. The tricolor, eglanterina, and nevadensis groups are more distinctive than the electra, burnsi, or diana groups, but all are closely related. Species groups are used to exemplify evolutionary trends within this large but cohesive genus. The saturniid fauna of the western United States is dominated by moths of the tribe Hemileucinae. Three genera in this tribe commonly occur north of Mexico: Hemileuca, Coloradia, and Automeris. Al though no Automeris are native to California about 50% of the Hemi leuca and Coloradia species in the United States occur in the state. The absence of Automeris and other species from California is due to the state's effective isolation from southern Arizona and mainland Mex ico by harsh mountains, deserts, the Gulf of California, and climatic differences. The Hemileuca of northern Arizona, Nevada, and Utah are very similar to that of California, while those of Oregon, Washing ton, and Idaho represent subsets of the northern California fauna. The majority of the saturniid species in the United States have had little or no impact on man, but some Hemileucinae have been of eco nomic importance. -

Bogbean Buckmoth (Hemileuca Sp.) in Canada
PROPOSED Species at Risk Act Recovery Strategy Series Adopted under Section 44 of SARA Recovery Strategy for the Bogbean Buckmoth (Hemileuca sp.) in Canada Bogbean Buckmoth 2015 1 Recommended citation: Environment Canada. 2015. Recovery Strategy for the Bogbean Buckmoth (Hemileuca sp.) in Canada [Proposed]. Species at Risk Act Recovery Strategy Series. Environment Canada, Ottawa. 18 pp. + Appendix. For copies of the recovery strategy, or for additional information on species at risk, including COSEWIC Status Reports, residence descriptions, action plans, and other related recovery documents, please visit the Species at Risk Public Registry1. Cover illustration: © Don Cuddy Également disponible en français sous le titre « Programme de rétablissement de l’hémileucin du ményanthe (Hemileuca sp.) au Canada [Proposition] » © Her Majesty the Queen in Right of Canada, represented by the Minister of the Environment, 2015. All rights reserved. ISBN Catalogue no. Content (excluding the illustrations) may be used without permission, with appropriate credit to the source. 1 http://sararegistry.gc.ca/default.asp?lang=En&n=24F7211B-1 RECOVERY STRATEGY FOR THE BOGBEAN BUCKMOTH (Hemileuca sp.) IN CANADA 2015 Under the Accord for the Protection of Species at Risk (1996), the federal, provincial, and territorial governments agreed to work together on legislation, programs, and policies to protect wildlife species at risk throughout Canada. In the spirit of cooperation of the Accord, the Government of Ontario has given permission to the Government of Canada to adopt the Recovery Strategy for Bogbean Buckmoth (Hemileuca sp.) in Ontario (Part 2) under Section 44 of the Species at Risk Act. Environment Canada has included an addition (Part 1) which completes the SARA requirements for this recovery strategy. -

Protection of Pandora Moth (Coloradia Pandora Blake) Eggs from Consumption by Golden-Mantled Ground Squirrels (Spermophilus Lateralis Say)
AN ABSTRACT OF THE THESIS OF Elizabeth Ann Gerson for the degree of Master of Science in Forest Science presented on 10 January, 1995. Title: Protection of Pandora Moth (Coloradia pandora Blake) Eggs From Consumption by Golden-mantled Ground Squirrels (Spermophilus lateralis Say) Abstract approved: Redacted for Privacy William C. McComb Endemic populations of pandora moths (Coloradia pandora Blake), a defoliator of western pine forests, proliferated to epidemic levels in central Oregon in 1986 and increased dramatically through 1994. Golden-mantled ground squirrels (Spermophilus lateralis Say) consume adult pandora moths, but reject nutritionally valuable eggs from gravid females. Feeding trials with captive S. lateralis were conducted to identify the mode of egg protection. Chemical constituents of fertilized eggs were separated through a polarity gradient of solvent extractions. Consumption of the resulting hexane, dichloromethane, and water egg fractions, and the extracted egg tissue residue, was evaluated by randomized 2-choice feeding tests. Consumption of four physically distinct egg fractions (whole eggs, "whole" egg shells, ground egg shells, and egg contents) also was evaluated. These bioassays indicated that C. pandora eggs are not protected chemically, however, the egg shell does inhibit S. lateralis consumption. Egg protection is one mechanism that enables C. pandora to persist within the forest food web. Spermophilus lateralis, a common and often abundant rodent of central Oregon pine forests, is a natural enemy of C. pandora -
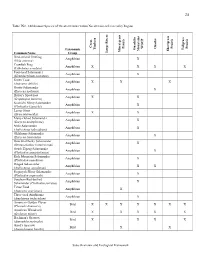
State Overview and Ecological Framework Table IN2. Oklahoma's
24 Table IN2. Oklahoma's Species of Greatest Conservation Need Cross-referenced by Region grass - Cross Prairie Prairie Prairie WGCP Ozarks Timbers Tallgrass Taxonomic Ouachita Mountains Shortgrass Mixed Common Name Group Large Rivers Bird-voiced Treefrog Amphibian X (Hyla avivoca) Crawfish Frog Amphibian X X X X (Lithobates areolata) Four-toed Salamander Amphibian X (Hemidactylium scutatum) Green Toad Amphibian X X X (Anaxyrus debilis) Grotto Salamander Amphibian X (Eurycea spelaeus) Hurter's Spadefoot Amphibian X X (Scaphiopus hurterii) Kiamichi Slimy Salamander Amphibian X (Plethodon kiamichi) Lesser Siren Amphibian X X (Siren intermedia) Many-ribbed Salamander Amphibian X (Eurycea multiplicata) Mole Salamander Amphibian X (Ambystoma talpoideum) Oklahoma Salamander Amphibian X (Eurycea tynerensis) Ouachita Dusky Salamander Amphibian X (Desmognathus brimleyorum) Ozark Zigzag Salamander Amphibian X (Plethodon angusticlavius) Rich Mountain Salamander Amphibian X (Plethodon ouachitae) Ringed Salamander Amphibian X X (Ambystoma annulatum) Sequoyah Slimy Salamander Amphibian X (Plethodon sequoyah) Southern Red-backed Amphibian X Salamander (Plethodon serratus) Texas Toad Amphibian X (Anaxyrus speciosus) Three-toed Amphiuma Amphibian X (Amphiuma tridactylum) American Golden Plover Bird X X X X X X X (Pluvialis dominica) American Woodcock Bird X X X X X (Scolopax minor) Bachman's Sparrow Bird X X X X (Aimophila aestivalis) Baird's Sparrow Bird X X (Ammodramus bairdii) State Overview and Ecological Framework 25 grass - ntains Cross Large Rivers -

The Taxonomic Report of the INTERNATIONAL LEPIDOPTERA SURVEY
Volume 8 Number 5 1 April, 2020 The Taxonomic Report OF THE INTERNATIONAL LEPIDOPTERA SURVEY ISSN 2643-4776 (print) / ISSN 2643-4806 (online) A phenotypic comparison of regional populations of Hemileuca maia (Drury, 1773) with designations of new subspecies (Bombycoidea, Saturniidae, Hemileucinae). Harry Pavulaan 606 Hunton Place NE Leesburg, VA. 20176 [email protected] ABSTRACT. Following refinement of the type locality of Hemileuca maia to the Long Island Pine Barrens of New York State by the author (Pavulaan, 2020), an evaluation of phenotypic characters of regional populations of H. maia is presented. The Long Island population is the nominotypical subspecies. Populations in southeastern coastal New England and offshore islands are presently considered nominotypical maia. However, several continental inland populations show evidence of subspecific variation. Four new subspecies are designated. Detailed phenotypic information of other interior regions is lacking. Additional key words: Pitch Pine Barrens, Scrub Oak Plains, isolate, Menyanthes trifoliata. ZooBank registration: urn:1sid:zoobank.org:pub:3595D21C-4FDE-4336-A588-4E68195E1118 INTRODUCTION The Buckmoths of North America are a bewildering blend of intergrading phenotypes that have been the subject of numerous studies (Ferguson, 1971; Tuskeset al., 1996; Rubinoffet al., 2017; Dupuiset al., 2018). Results of these studies are inconclusive over where to draw taxonomc limits. Michener (1952) proposed a subdivision of genus Hemileuca into four subgenera: Hemileuca (Walker, 1855), Pseudohazis (Grote & Robinson, 1866), Euleucophaeus (Packard, 1872) and Argyrauges (Grote, 1882). Nestled within subgenus Hemileuca is the Hemileuca maia complex, presently considered to be a closely- related group of species and unnamed populations of species H. maia. This group is characterized by variation in ground color (gray to black), bold median bands (white to yellow), and scale translucence. -

PORTABLE OUTDOOR CAGES for MATING FEMALE GIANT SILKWORM MOTHS (SATURNIIDAE)L
VOLUME 30, NUMBER 2 95 PORTABLE OUTDOOR CAGES FOR MATING FEMALE GIANT SILKWORM MOTHS (SATURNIIDAE)l THOMAS A. MILLER 2 AND WILLIAM J. COOPER ~ U. S. Army Medical Bioengineering Research and Development Laboratory, USAMBRDL BLDG 568, Fort Detrick, Maryland 21701 Like many lepidopterists who colonize and study the saturniids, we must find time apart from professional duties and other daily activities to pursue our interest in this family of moths. Therefore, our rearing methods must be both efficient and reliable if we are to accomplish any thing other than the mere maintenance of colonies. Although most of the saturniid species we have reared can be colonized for many genera tions without difficulty, we have at times had a particular problem obtain ing fertile eggs to perpetuate existing colonies or to begin new ones. This problem has been a function largely of limited available time to ensure the mating of females either by hand mating or by the use of indoor cages. For this reason we undertook an evaluation of methods for the routine, unattended mating of female saturniids indigenous to our area. We decided beforehand that the tying out of virgin females and the use of various-size stationary cages or traps (Collins & Weast, 1961; Quesada, 1967; Villiard, 1969) were not suitable to meet our requirements. Our approach, therefore, was to construct and test a series of portable out door cages that could be substituted locally for tying out or stationary cages and could also be easily transported for use in remote field areas. These cages were designed to minimize or prevent the escape of females placed therein while permitting pheromone-seeking males access for the purpose of copulation. -

Refereed Journal Articles at Vernon – Entomology 1975 1977 1978
Refereed Journal Articles at Vernon – Entomology 1975 Rogers, C.E., and J.C. Garrison. 1975. Seed destruction in Indigobush amorpha by a seed beetle. Journal of range Management 28: 241-242. Rogers, C.E., W.E. Clark, and H.R. Burke. 1975. Bionomics of Sibinia sulcatula (Coleoptera: Curculionidea) on mesquite in Texas. Southwestern Naturalist 20: 303-314. 1977 Rogers, C.E. 1977. Hosts and parasitoids of the Cecidomyiidae (Diptera) in the Rolling Plains of Texas. Journal of the Kansas Entomological Society 50: 179-186. Rogers, C.E., and N.V. Horner. 1977. Spiders of guar in Texas and Oklahoma. Environmental Entomology 6: 523-524. 1978 Slosser, J.E. 1978. Influence of planting date on boll weevil management. Southwestern Entomologist 3(3): 241-246. Slosser, J.E., J.R. Phillips, and G.A. Herzog. 1978. Bollworm damage and population development in relation to phenology of the cotton plant. Environmental Entomology 7(1): 144- 148. Slosser, J.E., and C.E. Rogers. 1978. A sequential sampling plan for midges (Diptera: Cecidomyiidae) infesting buds of guar. Journal of the Kansas Entomological Society 51(3): 499- 503. 1979 Wellik, M.J., J.E. Slosser, and R.D. Kirby. 1979. Evaluation of procedures for sampling Heliothis zea and Keiferia lycopersicella on tomatoes. Journal of Economic Entomology 72(5): 777-780. 1 1980 Slosser, J.E. 1980. Irrigation timing for bollworm management in cotton. Journal of Economic Entomology 73(2): 346-349. Slosser, J.E., and E.P. Boring, III. 1980. Shelterbelts and boll weevils: a control strategy based on management of overwintering habit. Environmental Entomology 9(1):1-6. -

NEWSLETTER• of the MICHIGAN ENTOMOLOGICAL SOCIETY
NEWSLETTER• of the MICHIGAN ENTOMOLOGICAL SOCIETY Volume 38, Numbers 4 December, 1993 Impacts ofBt on Non-Target Lepidoptera John W. Peacock, David L. Wagner, and Dale F. Schweitzer USDA Forest Service, Hamden, CT; University of Connecticut, Storrs, CT; and The Nature Conservancy, Port Norris, NT, respectively Introduction gypsy moth in Oregon. Sample et a1. ing attempts bycertain birds. In another (1 993) have likewise reported a signifi study, Bellocq et al. (1992) showed that Bacillus thuringiensis Berliner var. cant reduction inspecies abundance and the use of Btk increased immigration kurstaki (Btk) is one of the pesticides richness in non-target Lepidoptera in rates andcaused d ietary shifts inshrews. most commonly employed against lepi field studies in eastern West Virginia. We report here a summary of our dopteran forest pests. In the eastern U.S., James et al. (1993) haveshown thatBtk is studies aimed at determining the effect where millionsofhectares of deciduous toxic to late, but not early, instar larvae of Btko n non-target Lepidoptera inboth forest have been defoliated by the ''Eu of the beneficial cinnabar moth, Tyria laboratoryand field studies. Laboratory ropean" gypsy moth, Lymantria dispar jacobaeae (L.). bioassays were conducted on larvae in (L.), Btk has been used extenSively to In addition to its direct effects on seven families of native eastern U.S. slow the spread of this pest and to re native Lepidoptera, Btk can indirectly Macrolepidoptera. Field studies were duce defoliation. In 1992 alone, over affect other animals that rely on lepi carried out in Rockbridge County, Vir 300,000 ha were treated with Btk, in dopterous larvae as a primary source of ginia, and were the first to evaluate non cluding gypsy moth suppression activi food. -

First Record of Citheronia Regalis (Lepidoptera: Saturniidae) Feeding on Cotinus Obovatus (Anacardiaceae) Author(S): Gary R
First Record of Citheronia regalis (Lepidoptera: Saturniidae) Feeding on Cotinus obovatus (Anacardiaceae) Author(s): Gary R. Graves Source: Florida Entomologist, 100(2):474-475. Published By: Florida Entomological Society https://doi.org/10.1653/024.100.0210 URL: http://www.bioone.org/doi/full/10.1653/024.100.0210 BioOne (www.bioone.org) is a nonprofit, online aggregation of core research in the biological, ecological, and environmental sciences. BioOne provides a sustainable online platform for over 170 journals and books published by nonprofit societies, associations, museums, institutions, and presses. Your use of this PDF, the BioOne Web site, and all posted and associated content indicates your acceptance of BioOne’s Terms of Use, available at www.bioone.org/page/terms_of_use. Usage of BioOne content is strictly limited to personal, educational, and non-commercial use. Commercial inquiries or rights and permissions requests should be directed to the individual publisher as copyright holder. BioOne sees sustainable scholarly publishing as an inherently collaborative enterprise connecting authors, nonprofit publishers, academic institutions, research libraries, and research funders in the common goal of maximizing access to critical research. Scientific Notes First record of Citheronia regalis (Lepidoptera: Saturniidae) feeding on Cotinus obovatus (Anacardiaceae) Gary R. Graves1,2,* The regal moth (Citheronia regalis F.; Lepidoptera: Saturniidae) 2016) shows historic and recent records of C. regalis for only 11 of was historically distributed in eastern North America from southern the 34 counties in which natural populations of smoketree have been New England and southern Michigan, south to southern Florida, and documented (Davis & Graves 2016). west to eastern Nebraska and eastern Texas (Tuskes et al. -

Clicking Caterpillars: Acoustic Aposematism in Antheraea Polyphemus and Other Bombycoidea Sarah G
993 The Journal of Experimental Biology 210, 993-1005 Published by The Company of Biologists 2007 doi:10.1242/jeb.001990 Clicking caterpillars: acoustic aposematism in Antheraea polyphemus and other Bombycoidea Sarah G. Brown1, George H. Boettner2 and Jayne E. Yack1,* 1Department of Biology, Carleton University, Ottawa, Ontario, K1S 5B6, Canada and 2Plant Soil and Insect Sciences, University of Massachusetts, Amherst, MA 01003, USA *Author for correspondence (e-mail: [email protected]) Accepted 21 November 2006 Summary Acoustic signals produced by caterpillars have been correlated sound production with attack, and an increase documented for over 100 years, but in the majority of in attack rate was positively correlated with the number of cases their significance is unknown. This study is the first signals produced. In addition, sound production typically to experimentally examine the phenomenon of audible preceded or accompanied defensive regurgitation. sound production in larval Lepidoptera, focusing on a Bioassays with invertebrates (ants) and vertebrates (mice) common silkmoth caterpillar, Antheraea polyphemus revealed that the regurgitant is deterrent to would-be (Saturniidae). Larvae produce airborne sounds, predators. Comparative evidence revealed that other resembling ‘clicks’, with their mandibles. Larvae typically Bombycoidea species, including Actias luna (Saturniidae) signal multiple times in quick succession, producing trains and Manduca sexta (Sphingidae), also produce airborne that last over 1·min and include 50–55 clicks. Individual sounds upon attack, and that these sounds precede clicks within a train are on average 24.7·ms in duration, regurgitation. The prevalence and adaptive significance of often consisting of multiple components. Clicks are audible warning sounds in caterpillars is discussed. -

Insects That Feed on Trees and Shrubs
INSECTS THAT FEED ON COLORADO TREES AND SHRUBS1 Whitney Cranshaw David Leatherman Boris Kondratieff Bulletin 506A TABLE OF CONTENTS DEFOLIATORS .................................................... 8 Leaf Feeding Caterpillars .............................................. 8 Cecropia Moth ................................................ 8 Polyphemus Moth ............................................. 9 Nevada Buck Moth ............................................. 9 Pandora Moth ............................................... 10 Io Moth .................................................... 10 Fall Webworm ............................................... 11 Tiger Moth ................................................. 12 American Dagger Moth ......................................... 13 Redhumped Caterpillar ......................................... 13 Achemon Sphinx ............................................. 14 Table 1. Common sphinx moths of Colorado .......................... 14 Douglas-fir Tussock Moth ....................................... 15 1. Whitney Cranshaw, Colorado State University Cooperative Extension etnomologist and associate professor, entomology; David Leatherman, entomologist, Colorado State Forest Service; Boris Kondratieff, associate professor, entomology. 8/93. ©Colorado State University Cooperative Extension. 1994. For more information, contact your county Cooperative Extension office. Issued in furtherance of Cooperative Extension work, Acts of May 8 and June 30, 1914, in cooperation with the U.S. Department of Agriculture, -

Integrated Management Guidelines for Four Habitats and Associated
Integrated Management Guidelines for Four Habitats and Associated State Endangered Plants and Wildlife Species of Greatest Conservation Need in the Skylands and Pinelands Landscape Conservation Zones of the New Jersey State Wildlife Action Plan Prepared by Elizabeth A. Johnson Center for Biodiversity and Conservation American Museum of Natural History Central Park West at 79th Street New York, NY 10024 and Kathleen Strakosch Walz New Jersey Natural Heritage Program New Jersey Department of Environmental Protection State Forestry Services Office of Natural Lands Management 501 East State Street, 4th Floor MC501-04, PO Box 420 Trenton, NJ 08625-0420 For NatureServe 4600 N. Fairfax Drive – 7th Floor Arlington, VA 22203 Project #DDF-0F-001a Doris Duke Charitable Foundation (Plants) June 2013 NatureServe # DDCF-0F-001a Integrated Management Plans for Four Habitats in NJ SWAP Page | 1 Acknowledgments: Many thanks to the following for sharing their expertise to review and discuss portions of this report: Allen Barlow, John Bunnell, Bob Cartica, Dave Jenkins, Sharon Petzinger, Dale Schweitzer, David Snyder, Mick Valent, Sharon Wander, Wade Wander, Andy Windisch, and Brian Zarate. This report should be cited as follows: Johnson, Elizabeth A. and Kathleen Strakosch Walz. 2013. Integrated Management Guidelines for Four Habitats and Associated State Endangered Plants and Wildlife Species of Greatest Conservation Need in the Skylands and Pinelands Landscape Conservation Zones of the New Jersey State Wildlife Action Plan. American Museum of Natural History, Center for Biodiversity and Conservation and New Jersey Department of Environmental Protection, Natural Heritage Program, for NatureServe, Arlington, VA. 149p. NatureServe # DDCF-0F-001a Integrated Management Plans for Four Habitats in NJ SWAP Page | 2 TABLE OF CONTENTS Page Project Summary………………………………………………………………………………………..….