The Taxonomic Report of the INTERNATIONAL LEPIDOPTERA SURVEY
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Biology and Distribution of California Hemileucinae (Saturniidae)
Journal of the Lepidopterists' Society 38(4), 1984,281-309 THE BIOLOGY AND DISTRIBUTION OF CALIFORNIA HEMILEUCINAE (SATURNIIDAE) PAUL M. TUSKES 7900 Cambridge 141G, Houston, Texas 77054 ABSTRACT. The distribution, biology, and larval host plants for the 14 species and subspecies of California Hemileucinae are discussed in detail. In addition, the immature stages of Hemileuca neumogeni and Coloradia velda are described for the first time. The relationships among the Hemileuca are examined with respect to six species groups, based on adult and larval characters, host plant relationships and pheromone interactions. The tricolor, eglanterina, and nevadensis groups are more distinctive than the electra, burnsi, or diana groups, but all are closely related. Species groups are used to exemplify evolutionary trends within this large but cohesive genus. The saturniid fauna of the western United States is dominated by moths of the tribe Hemileucinae. Three genera in this tribe commonly occur north of Mexico: Hemileuca, Coloradia, and Automeris. Al though no Automeris are native to California about 50% of the Hemi leuca and Coloradia species in the United States occur in the state. The absence of Automeris and other species from California is due to the state's effective isolation from southern Arizona and mainland Mex ico by harsh mountains, deserts, the Gulf of California, and climatic differences. The Hemileuca of northern Arizona, Nevada, and Utah are very similar to that of California, while those of Oregon, Washing ton, and Idaho represent subsets of the northern California fauna. The majority of the saturniid species in the United States have had little or no impact on man, but some Hemileucinae have been of eco nomic importance. -

Bogbean Buckmoth (Hemileuca Sp.) in Canada
PROPOSED Species at Risk Act Recovery Strategy Series Adopted under Section 44 of SARA Recovery Strategy for the Bogbean Buckmoth (Hemileuca sp.) in Canada Bogbean Buckmoth 2015 1 Recommended citation: Environment Canada. 2015. Recovery Strategy for the Bogbean Buckmoth (Hemileuca sp.) in Canada [Proposed]. Species at Risk Act Recovery Strategy Series. Environment Canada, Ottawa. 18 pp. + Appendix. For copies of the recovery strategy, or for additional information on species at risk, including COSEWIC Status Reports, residence descriptions, action plans, and other related recovery documents, please visit the Species at Risk Public Registry1. Cover illustration: © Don Cuddy Également disponible en français sous le titre « Programme de rétablissement de l’hémileucin du ményanthe (Hemileuca sp.) au Canada [Proposition] » © Her Majesty the Queen in Right of Canada, represented by the Minister of the Environment, 2015. All rights reserved. ISBN Catalogue no. Content (excluding the illustrations) may be used without permission, with appropriate credit to the source. 1 http://sararegistry.gc.ca/default.asp?lang=En&n=24F7211B-1 RECOVERY STRATEGY FOR THE BOGBEAN BUCKMOTH (Hemileuca sp.) IN CANADA 2015 Under the Accord for the Protection of Species at Risk (1996), the federal, provincial, and territorial governments agreed to work together on legislation, programs, and policies to protect wildlife species at risk throughout Canada. In the spirit of cooperation of the Accord, the Government of Ontario has given permission to the Government of Canada to adopt the Recovery Strategy for Bogbean Buckmoth (Hemileuca sp.) in Ontario (Part 2) under Section 44 of the Species at Risk Act. Environment Canada has included an addition (Part 1) which completes the SARA requirements for this recovery strategy. -

Protection of Pandora Moth (Coloradia Pandora Blake) Eggs from Consumption by Golden-Mantled Ground Squirrels (Spermophilus Lateralis Say)
AN ABSTRACT OF THE THESIS OF Elizabeth Ann Gerson for the degree of Master of Science in Forest Science presented on 10 January, 1995. Title: Protection of Pandora Moth (Coloradia pandora Blake) Eggs From Consumption by Golden-mantled Ground Squirrels (Spermophilus lateralis Say) Abstract approved: Redacted for Privacy William C. McComb Endemic populations of pandora moths (Coloradia pandora Blake), a defoliator of western pine forests, proliferated to epidemic levels in central Oregon in 1986 and increased dramatically through 1994. Golden-mantled ground squirrels (Spermophilus lateralis Say) consume adult pandora moths, but reject nutritionally valuable eggs from gravid females. Feeding trials with captive S. lateralis were conducted to identify the mode of egg protection. Chemical constituents of fertilized eggs were separated through a polarity gradient of solvent extractions. Consumption of the resulting hexane, dichloromethane, and water egg fractions, and the extracted egg tissue residue, was evaluated by randomized 2-choice feeding tests. Consumption of four physically distinct egg fractions (whole eggs, "whole" egg shells, ground egg shells, and egg contents) also was evaluated. These bioassays indicated that C. pandora eggs are not protected chemically, however, the egg shell does inhibit S. lateralis consumption. Egg protection is one mechanism that enables C. pandora to persist within the forest food web. Spermophilus lateralis, a common and often abundant rodent of central Oregon pine forests, is a natural enemy of C. pandora -
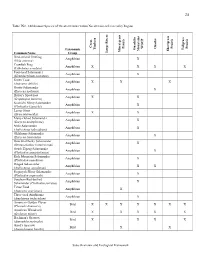
State Overview and Ecological Framework Table IN2. Oklahoma's
24 Table IN2. Oklahoma's Species of Greatest Conservation Need Cross-referenced by Region grass - Cross Prairie Prairie Prairie WGCP Ozarks Timbers Tallgrass Taxonomic Ouachita Mountains Shortgrass Mixed Common Name Group Large Rivers Bird-voiced Treefrog Amphibian X (Hyla avivoca) Crawfish Frog Amphibian X X X X (Lithobates areolata) Four-toed Salamander Amphibian X (Hemidactylium scutatum) Green Toad Amphibian X X X (Anaxyrus debilis) Grotto Salamander Amphibian X (Eurycea spelaeus) Hurter's Spadefoot Amphibian X X (Scaphiopus hurterii) Kiamichi Slimy Salamander Amphibian X (Plethodon kiamichi) Lesser Siren Amphibian X X (Siren intermedia) Many-ribbed Salamander Amphibian X (Eurycea multiplicata) Mole Salamander Amphibian X (Ambystoma talpoideum) Oklahoma Salamander Amphibian X (Eurycea tynerensis) Ouachita Dusky Salamander Amphibian X (Desmognathus brimleyorum) Ozark Zigzag Salamander Amphibian X (Plethodon angusticlavius) Rich Mountain Salamander Amphibian X (Plethodon ouachitae) Ringed Salamander Amphibian X X (Ambystoma annulatum) Sequoyah Slimy Salamander Amphibian X (Plethodon sequoyah) Southern Red-backed Amphibian X Salamander (Plethodon serratus) Texas Toad Amphibian X (Anaxyrus speciosus) Three-toed Amphiuma Amphibian X (Amphiuma tridactylum) American Golden Plover Bird X X X X X X X (Pluvialis dominica) American Woodcock Bird X X X X X (Scolopax minor) Bachman's Sparrow Bird X X X X (Aimophila aestivalis) Baird's Sparrow Bird X X (Ammodramus bairdii) State Overview and Ecological Framework 25 grass - ntains Cross Large Rivers -

Refereed Journal Articles at Vernon – Entomology 1975 1977 1978
Refereed Journal Articles at Vernon – Entomology 1975 Rogers, C.E., and J.C. Garrison. 1975. Seed destruction in Indigobush amorpha by a seed beetle. Journal of range Management 28: 241-242. Rogers, C.E., W.E. Clark, and H.R. Burke. 1975. Bionomics of Sibinia sulcatula (Coleoptera: Curculionidea) on mesquite in Texas. Southwestern Naturalist 20: 303-314. 1977 Rogers, C.E. 1977. Hosts and parasitoids of the Cecidomyiidae (Diptera) in the Rolling Plains of Texas. Journal of the Kansas Entomological Society 50: 179-186. Rogers, C.E., and N.V. Horner. 1977. Spiders of guar in Texas and Oklahoma. Environmental Entomology 6: 523-524. 1978 Slosser, J.E. 1978. Influence of planting date on boll weevil management. Southwestern Entomologist 3(3): 241-246. Slosser, J.E., J.R. Phillips, and G.A. Herzog. 1978. Bollworm damage and population development in relation to phenology of the cotton plant. Environmental Entomology 7(1): 144- 148. Slosser, J.E., and C.E. Rogers. 1978. A sequential sampling plan for midges (Diptera: Cecidomyiidae) infesting buds of guar. Journal of the Kansas Entomological Society 51(3): 499- 503. 1979 Wellik, M.J., J.E. Slosser, and R.D. Kirby. 1979. Evaluation of procedures for sampling Heliothis zea and Keiferia lycopersicella on tomatoes. Journal of Economic Entomology 72(5): 777-780. 1 1980 Slosser, J.E. 1980. Irrigation timing for bollworm management in cotton. Journal of Economic Entomology 73(2): 346-349. Slosser, J.E., and E.P. Boring, III. 1980. Shelterbelts and boll weevils: a control strategy based on management of overwintering habit. Environmental Entomology 9(1):1-6. -

NEWSLETTER• of the MICHIGAN ENTOMOLOGICAL SOCIETY
NEWSLETTER• of the MICHIGAN ENTOMOLOGICAL SOCIETY Volume 38, Numbers 4 December, 1993 Impacts ofBt on Non-Target Lepidoptera John W. Peacock, David L. Wagner, and Dale F. Schweitzer USDA Forest Service, Hamden, CT; University of Connecticut, Storrs, CT; and The Nature Conservancy, Port Norris, NT, respectively Introduction gypsy moth in Oregon. Sample et a1. ing attempts bycertain birds. In another (1 993) have likewise reported a signifi study, Bellocq et al. (1992) showed that Bacillus thuringiensis Berliner var. cant reduction inspecies abundance and the use of Btk increased immigration kurstaki (Btk) is one of the pesticides richness in non-target Lepidoptera in rates andcaused d ietary shifts inshrews. most commonly employed against lepi field studies in eastern West Virginia. We report here a summary of our dopteran forest pests. In the eastern U.S., James et al. (1993) haveshown thatBtk is studies aimed at determining the effect where millionsofhectares of deciduous toxic to late, but not early, instar larvae of Btko n non-target Lepidoptera inboth forest have been defoliated by the ''Eu of the beneficial cinnabar moth, Tyria laboratoryand field studies. Laboratory ropean" gypsy moth, Lymantria dispar jacobaeae (L.). bioassays were conducted on larvae in (L.), Btk has been used extenSively to In addition to its direct effects on seven families of native eastern U.S. slow the spread of this pest and to re native Lepidoptera, Btk can indirectly Macrolepidoptera. Field studies were duce defoliation. In 1992 alone, over affect other animals that rely on lepi carried out in Rockbridge County, Vir 300,000 ha were treated with Btk, in dopterous larvae as a primary source of ginia, and were the first to evaluate non cluding gypsy moth suppression activi food. -

Moths of Ohio Guide
MOTHS OF OHIO field guide DIVISION OF WILDLIFE This booklet is produced by the ODNR Division of Wildlife as a free publication. This booklet is not for resale. Any unauthorized INTRODUCTION reproduction is prohibited. All images within this booklet are copyrighted by the Division of Wildlife and it’s contributing artists and photographers. For additional information, please call 1-800-WILDLIFE. Text by: David J. Horn Ph.D Moths are one of the most diverse and plentiful HOW TO USE THIS GUIDE groups of insects in Ohio, and the world. An es- Scientific Name timated 160,000 species have thus far been cata- Common Name Group and Family Description: Featured Species logued worldwide, and about 13,000 species have Secondary images 1 Primary Image been found in North America north of Mexico. Secondary images 2 Occurrence We do not yet have a clear picture of the total Size: when at rest number of moth species in Ohio, as new species Visual Index Ohio Distribution are still added annually, but the number of species Current Page Description: Habitat & Host Plant is certainly over 3,000. Although not as popular Credit & Copyright as butterflies, moths are far more numerous than their better known kin. There is at least twenty Compared to many groups of animals, our knowledge of moth distribution is very times the number of species of moths in Ohio as incomplete. Many areas of the state have not been thoroughly surveyed and in some there are butterflies. counties hardly any species have been documented. Accordingly, the distribution maps in this booklet have three levels of shading: 1. -

Saturniidae of 'Los Altos De Chiapas," Mexico (Lepidoptera: Bombycoidea)
Vol. 9 No. 1 1998 BEUTELSPACHER and BALCAZAR: Saturniidae of "Los Altos de Chiapas" 19 TROPICAL LEPIDOPTERA, 9(1): 19-22 SATURNIIDAE OF 'LOS ALTOS DE CHIAPAS," MEXICO (LEPIDOPTERA: BOMBYCOIDEA) CARLOS R. BEUTELSPACHER-BAIGTS AND MANUEL BALCAZAR-LARA Coleccion Nacional de Insectos, Instituto de Biologia, UNAM, A.P. 70-153, Mexico City, 04510 DF, Mexico ABSTRACT.- A faunal study for the family Saturniidae, of "Rancho Nuevo", San Cristobal de Las Casas, Chiapas, Mexico is presented in this paper. Thirteen species of nine genera were found in the area. The fauna is compared with those of other Mexican localities in published papers. RESUMEN.- Se estudiaron las mariposas de la familia Saturniidae, de "Rancho Nuevo", San Cristobal de Las Casas, Chiapas, Mexico, encontrandose 13 especies repartidas en nueve generos. Se compara esta fauna, con otras del pai's y se senalan los Indices de Similitud. KEY WORDS: Arsenurinae, biodiversity, Central America, Ceratocampinae, distribution, fauna, Hemileucinae, Mesoamerica, Neotropical, Saturniinae, zoogeography. This is the second of a series of papers on the Lepidoptera fauna RESULTS of "Rancho Nuevo," San Cristobal de las Casas, Chiapas, Mexico dealing with the family Saturniidae. The description of the study area A total of 13 species of 9 genera were found in the study area, 2 is as follows (see also Beutelspacher, 1995): location is in central of which are considered endemics to the area: Syssphinx gomezi Chiapas, at 16°40'13"N and 92°33'49"W. The climate in the area is Lemaire and Coloradia casanovai Beutelspacher. The months when subhumid temperate. Warmest months are June and July, with an adult specimens of the species were collected, and their number, are average temperatue 15.5°C; the coldest months are December and pointed out in the following list. -

(Hemileuca Nuttalli) and GROUND MANTID (Litaneutria Minor) SEARCHES in the SOUTH OKANAGAN VALLEY, BRITISH COLUMBIA, 2009
NUTTALL’S BUCKMOTH (Hemileuca nuttalli) AND GROUND MANTID (Litaneutria minor) SEARCHES IN THE SOUTH OKANAGAN VALLEY, BRITISH COLUMBIA, 2009 By Vicky Young and Dawn Marks, BC Conservation Corps BC Ministry of Environment Internal Working Report September 23, 2009 ii EXECUTIVE SUMMARY The Nuttall’s Buckmoth (Hemileuca nuttalli) and Ground Mantid (Litaneutria minor) are two invertebrate species listed for inventory under the BC Conservation Framework (2009). The Nuttall’s Buckmoth is listed as a mid priority species on The COSEWIC Candidate List (COSEWIC 2009). The Ground Mantid may be recommended as a COSEWIC candidate (Rob Cannings, COSEWIC Arthropod subcommittee, pers. comm.). These species were included in a list of target species for the BC Conservation Corps grassland species inventory crew. The crew spent 4 days surveying antelope-brush habitats within the Southern Okanagan between August 24th and September 1st 2009. These searches did not result in any detection of either target species. This preliminary attempt to address the lack of data for these invertebrate species will help inform future inventory efforts. ACKNOWLEDGMENTS Funding for this project was provided by the BC Ministry of Environment through the BC Conservation Corps and through the BC Conservation Framework. We appreciate administrative support from the BC Conservation Foundation (Barb Waters). Guidance and mentorship was provided by Orville Dyer, Wildlife Biologist with the BC Ministry of Environment. Training regarding moth behaviour and identification was provided by Dennis St. John. Rob Cannings, Curator of Entomology, Royal BC Museum, provided insect biodiversity, insect inventory, collection of voucher specimens and identification training. Jerry Mitchell and Aaron Reid, biologists with the BC Ministry of Environment, provided assistance with Wildlife Species Inventory database submissions. -

Denver Museum of Natural History
PROCEEDINGS OF THE Denver Museum of Natural History SERIES 3, NUMBER 3, OCTOBER 15, 1993 A REVIEW OF THE GENUS AGAPEMA (LEPIDOPTERA: SATURNIIDAE) RICHARD S. PEIGLER Department of Zoology, Denver Museum of Natural History 2001 Colorado Boulevard, Denver, Colorado 80205-5798 and ROY O. KENDALL 5598 Mt. McKinley Drive N.E. San Antonio, Texas 78251-3626 ABSTRACT — Agapema is a genus of saturniid moths ranging in Mexico and the southwestern United States. Agapema galbina, the type-species, was found to be misidenti- fied by authors during the last 21 years. John Pope collected the original type specimens in the lower Rio Grande Valley of Texas, not in western Texas. Agapema platensis, spec. nov., is described from the Edwards Plateau of Texas. Four taxa in far western Texas (dyari, nom. rev.), Arizona and western Mexico (anona, stat. rev.), Baja California (pelora, stat. nov.) and northeastern Mexico (dentifasciata, stat. nov.), previously considered to he subspecies of galbina, are elevated to full species rank based on larval and genitalic dif- ferences. The female and mature larva of dentifasciata and mature larvae of dyari and platensis are described for the first time. Figures and a key to the adult moths are pro- vided. The distribution of each species is plotted on a map. All known records for host- plants and parasitoids are tabulated, and phylogeny, habitats, and other field observa- tions are discussed. KEYWORDS: Agapema, Arizona, Colorado, Condalia, galbina, Mexico, moths, par- asitoids, Rhamnaceae, Saturnia, Saturniidae, taxonomy, Texas PEIGLER AND KENDALL The genus Agapema is a holophyletic group of TAMU— Texas A&M University, College small to medium-sized nocturnal saturniid moths that Station range in southwestern North America south to the region SDNHM— San Diego Natural History Museum, of Mexico City. -

A NEW SPECIES of HEMILEUCA from the SOUTHWESTERN UNITED STATES (SATURNIIDAE) the Genus H Emileuca Consists of 23 Described Speci
Journal of the Lepidopterists' Society 32(2), 1978, 97-102 A NEW SPECIES OF HEMILEUCA FROM THE SOUTHWESTERN UNITED STATES (SATURNIIDAE) PAUL M. TUSKES DepaJtment of Environmental Toxicology, University of California, Davis. Davis, California 95616 ABSTRACT. Hemileuca griHini Tuskes which occurs in southern Utah and northern Arizona was collected for the first time in 1974. The adult moth is a black and white day flying saturniid which is active during September and October. The larval hostplant is black brush, Coleogyne ramosissima. This species has a unique taxonomic position in that both the adult and larva exhibit morphological characters which are intermediate to the Pseudohazis and Hemileuca groups, thus, a continuum of characters exists between these two previously separated genera. The genus H emileuca consists of 23 described species, 16 of which have partial or complete distributional patterns north of Mexico. The moths within this genus are large to moderate in size, and exhibit a great deal of hostplant and habitat diversity. Adults are characterized by hav ing the labial palpi fused to each other forming a small unsegmented bilobed structure; also, the male has bipectinate antennae. Members of Coloradia, the genus most closely related to Hemileuca, have labial palpi which are separate, and males have antennae which are quadripectinate. The last Hemileuca described as a distinct species was chinatiensis (Tinkham), in 1943. The significance of H. chinatiensis as a species with genitalic characters intermediate between Pseudohazis and Hemi leuca was overlooked by Tinkham; not until Ferguson (1971) was its taxonomic position made clear. Michener (1962) combined the genera Pseudohazis and Hemileuca on the basis of their morphological similarity, but made no mention of chinatiensis. -

Hemileuca Lucina Henry Edwards, H. Nevadensis Stretch, Anisota Senatoria 0
Journal of the Lepidopterists' Society 38(1). 1984. 51-56 TWO INTERESTING ARTIFICIAL HYBRID CROSSES IN THE GENERA HEMILEUCA AND ANISOTA (SATURNIIDAE) RICHARD STEVEN PEIGLER1 303 Shannon Drive, Greenville, South Carolina 29615 AND BENJAMIN D. WILLIAMS The Lawrence Academy, Groton, Massachusetts 01450 ABSTRACT. Two crosses were reared to the adult stage with saturniid moths from different areas of the United States. These were Hemileuca lucina 5 x H. nevadensis Q reared in Massachusetts and Texas on Salix, and Anisata senataria 5 x A. aslari Q reared in Connecticut on Quercus caccinea. Larvae and adults of both crosses were interme diate. Descriptions and figures of the hybrids are given. Several isolating mechanisms between the parent species were tested and are discussed. Dozens of artificial crosses in the Saturniidae have been successfully reared since the previous century, but virtually all of these have in volved species of the subfamily Saturniinae. This paper deals with two remarkable crosses obtained by the junior author utilizing small satur niid moths belonging to the subfamilies Hemileucinae and Ceratocam pinae.2 In both crosses, species native to the Southwest were reared in the Northeast and females from those rearings attracted congeneric diurnal males native to the Northeast. The species involved were Hemileuca lucina Henry Edwards, H. nevadensis Stretch, Anisota senatoria 0. E. Smith) and A. oslari W. Rothschild. For information on the adult morphology, wing pattern, immature stages, hostplants, reproductive behavior, and geographical distributions of these four parent species, the reader is referred to works by Ferguson (1971) and Riotte and Peigler (1981). Hemileuca lucina <3 x H.