The Cardiac Output-Heart Rate Relationship Under Different Conditions
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Heart Rate Information Sheet Finding Your Pulse Taking Your Pulse
Heart rate In this activity you will measure your heart rate and investigate the effect that other things have on your heart rate. Information sheet You can measure your heart (pulse) rate anywhere on your body where a major artery is close to the surface of your skin. The easiest places are: • on the front of your forearm • just above your wrist on your thumb side • on the side of your neck about half way between your chin and your ear. Finding your pulse Try to locate your pulse in one of these places, using the tips of your index and middle fingers. You should feel a gentle, regular beat. This is your heart rate. Do not use your thumb, as your thumb has a pulse of its own. Taking your pulse When you find your pulse, use a stopwatch or a watch with a second hand to count how many beats there are in a full minute (60 seconds). In most situations, taking your pulse rate over one minute will give a reasonably accurate result, but if you want to take your pulse after exercise, you should do so over a much shorter time interval. After exercise your pulse rate will be changing rapidly. To get a reasonably accurate result, start to measure the rate immediately after the exercise and count the number of beats in 10 seconds. Multiply this number by six to find your heart rate. For an adult, a normal resting heart rate is between 60–100 beats a minute. The fitter you are, the lower your resting heart beat will be. -

Heart to Heart - STEAM Activity
Heart to Heart - STEAM Activity Purpose: The main objective of this exercise is to introduce how the human heart works. This lesson will be divided into four different lessons, including an introduction to heart anatomy, heart beats and pulses, and the circulatory system. We will be using coloring activities, stethoscopes, and handmade pumps to reinforce the concepts seen in this lesson. Vocabulary ● Artery: A blood vessel that carries blood high in oxygen content away from the heart to the farthest reaches of the body. ● Vein: a Blood vessel that carries blood low in oxygen content from the body back to the heart. ● Atrium: One of the two upper cavities of the heart that passes blood to the ventricles. ● Ventricles: One of the two lower chambers of the heart that receives blood from the atria. ● Valves: Tissue-paper thin membranes attached to the heart wall that constantly open and close to regulate blood flow. ● Pulse: A rhythmical, mechanical throbbing of the arteries as blood pumps through them. ● Heart Rate: The number of times per minute that the heart contracts - the number of heart beats per minute (bpm). ● Taquicardia: A high resting heart rate that is usually higher than 100 beats per minute. ● Bradycardia: A low resting heart rate that is usually lower than 60 beats per minute. ● Circulation: The movement of blood through the vessels of the body by the pumping action of the heart. It distributes nutrients and oxygen and removes waste products from all parts of the body. ● Pulmonary Circulation: The portion of the circulatory system that carries deoxygenated blood from the heart to the lungs and oxygenated blood back to the heart. -

Toolbox-Talks--Blood-Pressure.Pdf
TOOLBOX Toolbox Talk #1 TALKS Blood Pressure vs. Heart Rate While your blood pressure is the force of your blood moving through your blood vessels, your heart rate is the number of times your heart beats per minute. They are two separate measurements and indicators of health. • For people with high blood pressure (HBP or hypertension), there’s no substitute for measuring blood pressure. • Heart rate and blood pressure do not necessarily increase at the same rate. A rising heart rate does not cause your blood pressure to increase at the same Quarter: rate. Even though your heart is beating more times a minute, healthy blood BLOOD vessels dilate (get larger) to allow more blood to flow through more easily. PRESSURE When you exercise, your heart speeds up so more blood can reach your muscles. It may be possible for your heart rate to double safely, while your blood pressure may respond by only increasing a modest amount. Talk Number: Heart Rate and Exercise 1 In discussions about high blood pressure, you will often see heart rate Blood mentioned in relation to exercise. Your target heart rate is based on age and Pressure can help you monitor the intensity of your exercise. vs. • If you measure your heart rate (take your pulse) before, during and after Heart Rate physical activity, you’ll notice it will increase over the course of the exercise. • The greater the intensity of the exercise, the more your heart rate will increase. • When you stop exercising, your heart rate does not immediately return to your normal (resting) heart rate. -

Effects of Vasodilation and Arterial Resistance on Cardiac Output Aliya Siddiqui Department of Biotechnology, Chaitanya P.G
& Experim l e ca n i t in a l l C Aliya, J Clinic Experiment Cardiol 2011, 2:11 C f a Journal of Clinical & Experimental o r d l DOI: 10.4172/2155-9880.1000170 i a o n l o r g u y o J Cardiology ISSN: 2155-9880 Review Article Open Access Effects of Vasodilation and Arterial Resistance on Cardiac Output Aliya Siddiqui Department of Biotechnology, Chaitanya P.G. College, Kakatiya University, Warangal, India Abstract Heart is one of the most important organs present in human body which pumps blood throughout the body using blood vessels. With each heartbeat, blood is sent throughout the body, carrying oxygen and nutrients to all the cells in body. The cardiac cycle is the sequence of events that occurs when the heart beats. Blood pressure is maximum during systole, when the heart is pushing and minimum during diastole, when the heart is relaxed. Vasodilation caused by relaxation of smooth muscle cells in arteries causes an increase in blood flow. When blood vessels dilate, the blood flow is increased due to a decrease in vascular resistance. Therefore, dilation of arteries and arterioles leads to an immediate decrease in arterial blood pressure and heart rate. Cardiac output is the amount of blood ejected by the left ventricle in one minute. Cardiac output (CO) is the volume of blood being pumped by the heart, by left ventricle in the time interval of one minute. The effects of vasodilation, how the blood quantity increases and decreases along with the blood flow and the arterial blood flow and resistance on cardiac output is discussed in this reviewArticle. -

Jugular Venous Pressure
NURSING Jugular Venous Pressure: Measuring PRACTICE & SKILL What is Measuring Jugular Venous Pressure? Measuring jugular venous pressure (JVP) is a noninvasive physical examination technique used to indirectly measure central venous pressure(i.e., the pressure of the blood in the superior and inferior vena cava close to the right atrium). It is a part of a complete cardiovascular assessment. (For more information on cardiovascular assessment in adults, see Nursing Practice & Skill ... Physical Assessment: Performing a Cardiovascular Assessment in Adults ) › What: Measuring JVP is a screening mechanism to identify abnormalities in venous return, blood volume, and right heart hemodynamics › How: JVP is determined by measuring the vertical distance between the sternal angle and the highest point of the visible venous pulsation in the internal jugular vein orthe height of the column of blood in the external jugular vein › Where: JVP can be measured in inpatient, outpatient, and residential settings › Who: Nurses, nurse practitioners, physician assistants, and treating clinicians can measure JVP as part of a complete cardiovascular assessment What is the Desired Outcome of Measuring Jugular Venous Pressure? › The desired outcome of measuring JVP is to establish the patient’s JVP within the normal range or for abnormal JVP to be identified so that appropriate treatment may be initiated. Patients’ level of activity should not be affected by having had the JVP measured ICD-9 Why is Measuring Jugular Venous Pressure Important? 89.62 › The JVP is -
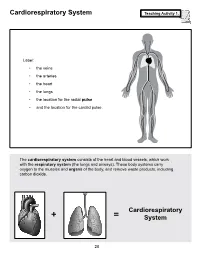
Cardiorespiratory System Teaching Activity 1
Cardiorespiratory System Teaching Activity 1. Label: • the veins • the arteries • the heart • the lungs • the location for the radial pulse • and the location for the carotid pulse. The cardiorespiratory system consists of the heart and blood vessels, which work with the respiratory system (the lungs and airways). These body systems carry oxygen to the muscles and organs of the body, and remove waste products, including carbon dioxide. Cardiorespiratory + = System 28 The Most Important Muscle: The Heart Teaching Activity 1, continued. In the circulatory system, the heart is a muscle that acts as a pump. In fact, the heart is a double pump. Blood that needs oxygen enters the heart and is pumped by the first pump to the lungs. The second pump of the heart pumps the oxygen-rich blood to all the other parts of the body. This gives the heart its common, “lub-dub” sound. The number of times the heart pumps, or beats, is counted in a minute. This is known as the pulse rate. A person’s pulse is affected by their current level of activity. If you are sleeping or do no physical activity at all, your heart is pumping at a resting heart rate. When active, you are using all your body systems. These systems require fuel in the form of calories (found in food), and oxygen (what you breathe). The more active you are, the Hearty Facts more fuel your muscles need. This is why your breathing rate and your heart rate increase when • Your system of blood vessels (arteries, you exercise. veins, and capillaries) is over 60,000 miles long. -

Cardiovascular System: Heart
Cardiovascular System: Heart Cardiovascular System – Heart Conducting cells: Cardiac Electrophysiology Cardiac cells specialized to quickly spread action potentials across myocardium • Weak force generators System allows for orderly, sequential depolarization and Intrinsic Conduction System: contraction of heart Normal sinus rhythm: 1) AP originates at SA node 2) SA node fires at 60 – 100 beats / min Atrial internodal tracts Sinoatrial node: (SA node) 3) Correct myocardial activation sequence • Located in right atrial wall • Initiates action potentials (APs) • Pacemaker (~ 80 beats / min) Bundle branches Atrioventricular node: (AV Node) • Connects atria to ventricles • Slowed conduction velocity • Ventricular filling Purkinje Bundle of His fibers Marieb & Hoehn (Human Anatomy and Physiology, 8th ed.) – Figure 18.14 1 Cardiovascular System – Heart Cardiac Electrophysiology The autonomic nervous system can directly affect the heart rate; these effects are called chronotropic effects Recall: spontaneous depolarization = VG Na+ channels Positive chronotrophic effects: (increase heart rate) • Under sympathetic control Leads to g ; cells SA Na NE node reach threshold more rapidly Sinoatrial node Pharmacology: β1 receptors β-blockers (e.g., propanolol) Negative chronotrophic effects: (decrease heart rate) • Under parasympathetic control Leads to gNa; cells reach threshold SA less rapidly ACh node Leads to gK; cells hyperpolarized during Muscarinic receptors repolarization stage (further from threshold) Costanzo (Physiology, 4th ed.) – Figure -

Q & a About Heart Rate Monitor Use and Assessment
Q & A About Heart Rate Monitor Use and Assessment As adults, we tend to think back to our personal experiences in P.E. class where the athletes set the bar for participation while the non-athletes struggled to keep up. As a result, some ended up hating P.E. class and physical activity because they could never run fast enough or jump high enough to achieve success. Since the mid 1990’s, physical education has been undergoing a major shift. The focus has moved from the assessment of performing sports skills toward maintaining or improving overall health related fitness. Of course we still use sport activities as a vehicle, but the focus is on functional movement and effectively increasing and maintaining heart rate, not on wins, losses or how many baskets you can make. As a result, we’ve begun to use technology to assist with the assessment of physical activity. We’ve received a lot of feedback regarding the use of heart rate monitors in P.E. classes, both positive and negative, so hopefully this Q&A form will help explain the process and procedure for the use of this type of equipment in our classes. As a district, we are dedicated to developing both the mind and bodies of our students so they may live an active and healthy lifestyle. We would like our students to have a strong understanding of exercise principles and how to accurately and safely apply them in their lives. Q: How often will my son/daughter use heart rate monitors in P.E. -

Cardiovascular System
Grades 3 to 5 • Human Body Series Cardiovascular System These activities will help your students learn about the heart and cardiovascular KidsHealth.org/classroom system. Teacher’s Guide Related KidsHealth Links This guide includes: Articles for Kids: Your Heart & Circulatory System • Standards KidsHealth.org/en/kids/heart.html • Related Links What's Blood? • Discussion Questions KidsHealth.org/en/kids/blood.html • Activities for Students Heart Disease KidsHealth.org/en/kids/heart-disease.html • Reproducible Materials I Had Heart Surgery: Noah's Story KidsHealth.org/en/kids/story-noah.html Video: Heart & Circulatory System Standards KidsHealth.org/kid/stay_healthy/fit/work_it_out.html This guide correlates with Quiz: Heart & Circulatory System the following National Health KidsHealth.org/en/kids/csquiz.html Education Standards: Activity: Heart & Circulatory System KidsHealth.org/en/kids/bfs-csactivity.html Students will: • Comprehend concepts related Word Find: Heart & Circulatory System to health promotion and KidsHealth.org/en/kids/bfs-cswordsearch.html disease prevention to enhance health. Words to Know (Heart Glossary) • Demonstrate the ability to KidsHealth.org/en/kids/heart-glossary.html access valid information and Why Exercise Is Cool products and services to KidsHealth.org/en/kids/work-it-out.html enhance health. • Demonstrate the ability to use Resources for Teachers: interpersonal communication Classroom Exercise Breaks for Elementary Students skills to enhance health and KidsHealth.org/en/parents/elementary-exercises.html avoid or reduce health risks. • Demonstrate the ability to Cardiomyopathy Special Needs Factsheet use decision-making skills to KidsHealth.org/en/parents/cardiomyopathy-factsheet.html enhance health. Congenital Heart Defects Special Needs Factsheet • Demonstrate the ability to use KidsHealth.org/en/parents/heart-defects-factsheet.html goal-setting skills to enhance health. -

Cardiovascular Responses to Hypoxemia in Sinoaortic-Denervated Fetal Sheep
003 1-399819 1 /3004-038 1$03.0010 PEDIATRIC RESEARCH Vol. 30. No. 4, I991 Copyright ID1991 International Pediatric Research Foundation. Inc. I1riiirc~c/it1 U.S. ,.I Cardiovascular Responses to Hypoxemia in Sinoaortic-Denervated Fetal Sheep JOSEPH ITSKOVITZ (ELDOR), EDMOND F. LAGAMMA. JAMES BRISTOW, AND ABRAHAM M. RUDOLPH Ccirdiovascz~karResearch Instillrle. Unlver:c.i/yqf Califi~rniu,Sari Francisco. Sun Francisco. Cu11fi)rilia94/43 ABSTRACT. Fetal cardiovascular response to acute hy- hypoxemia in postnatal life (1 3). The vascular effects of periph- poxemia is characterized by bradycardia, hypertension, and eral chemoreceptor stimulation, with ventilation held constant, redistribution of cardiac output. The role of aortic and include coronary vasodilation and vasoconstriction in the carotid chemoreceptors in mediating these responses was splanchnic organs and the skeletal muscles. Stimulation of the examined in eight sinoaortic-denervated and nine sham- carotid body chemoreceptors results in reflex bradycardia and operated fetal lambs. Blood gases, pH, heart rate, arterial negative inotropic responses. The bradycardia and peripheral pressure, and blood flow distribution were determined be- vasoconstriction during carotid chemoreceptor stimulation can fore and during hypoxemia. In intact fetuses, heart rate be reversed by effects arising from concurrent hypernea (13). fell from 184 -+ 12 to 165 + 23 beatslmin (p< 0.01) but The arterial chemoreceptors (aortic and carotid bodies) are increased from 184 + 22 to 200 + 16 beatslmin (p< 0.05) active in the fetal lamb and are responsive to hypoxemia (14- in the sinoaortic-denervated fetuses. Intact fetuses showed 21). Stimulation of the fetal arterial chemoreceptors result in an early hypertensive response to hypoxemia, whereas the bradycardia, which is abolished by SAD (19, 20, 22). -

Basic Cardiac Rhythms – Identification and Response Module 1 ANATOMY, PHYSIOLOGY, & ELECTRICAL CONDUCTION Objectives
Basic Cardiac Rhythms – Identification and Response Module 1 ANATOMY, PHYSIOLOGY, & ELECTRICAL CONDUCTION Objectives ▪ Describe the normal cardiac anatomy and physiology and normal electrical conduction through the heart. ▪ Identify and relate waveforms to the cardiac cycle. Cardiac Anatomy ▪ 2 upper chambers ▪ Right and left atria ▪ 2 lower chambers ▪ Right and left ventricle ▪ 2 Atrioventricular valves (Mitral & Tricuspid) ▪ Open with ventricular diastole ▪ Close with ventricular systole ▪ 2 Semilunar Valves (Aortic & Pulmonic) ▪ Open with ventricular systole ▪ Open with ventricular diastole The Cardiovascular System ▪ Pulmonary Circulation ▪ Unoxygenated – right side of the heart ▪ Systemic Circulation ▪ Oxygenated – left side of the heart Anatomy Coronary Arteries How The Heart Works Anatomy Coronary Arteries ▪ 2 major vessels of the coronary circulation ▪ Left main coronary artery ▪ Left anterior descending and circumflex branches ▪ Right main coronary artery ▪ The left and right coronary arteries originate at the base of the aorta from openings called the coronary ostia behind the aortic valve leaflets. Physiology Blood Flow Unoxygenated blood flows from inferior and superior vena cava Right Atrium Tricuspid Valve Right Ventricle Pulmonic Valve Lungs Through Pulmonary system Physiology Blood Flow Oxygenated blood flows from the pulmonary veins Left Atrium Mitral Valve Left Ventricle Aortic Valve Systemic Circulation ▪ Blood Flow Through The Heart ▪ Cardiology Rap Physiology ▪ Cardiac cycle ▪ Represents the actual time sequence between -

Pulse Wave Analysis to Estimate Cardiac Output
CLINICAL FOCUS REVIEW Jerrold H. Levy, M.D., F.A.H.A., F.C.C.M., Editor Pulse Wave Analysis to Estimate Cardiac Output Karim Kouz, M.D., Thomas W. L. Scheeren, M.D., Daniel de Backer, M.D., Bernd Saugel, M.D. ardiac output (CO)–guided therapy is a promising reference CO value measured using an indicator dilution Capproach to hemodynamic management in high-risk method (transpulmonary thermodilution or lithium dilu- patients having major surgery1 and in critically ill patients tion).5,9 CO measurement using indicator dilution methods with circulatory shock.2 Pulmonary artery thermodilu- requires a (central) venous catheter for indicator injection tion remains the clinical reference method for CO mea- upstream in the circulation and a dedicated arterial catheter Downloaded from http://pubs.asahq.org/anesthesiology/article-pdf/134/1/119/513741/20210100.0-00023.pdf by guest on 29 September 2021 surement,3 but the use of the pulmonary artery catheter and measurement system to detect downstream indicator decreased over the past two decades.4 Today, various CO temperature or concentration changes.5,9–11 monitoring methods with different degrees of invasiveness The VolumeView system (Edwards Lifesciences, are available, including pulse wave analysis.5 Pulse wave USA) and the PiCCO system (Pulsion Medical Systems, analysis is the mathematical analysis of the arterial blood Germany) calibrate pulse wave analysis–derived CO to pressure waveform and enables CO to be estimated con- transpulmonary thermodilution–derived CO. To measure tinuously and in real time.6 In this article, we review pulse CO using transpulmonary thermodilution, a bolus of cold wave analysis methods for CO estimation, including their crystalloid solution is injected in the central venous circu- underlying measurement principles and their clinical appli- lation.10 The cold indicator bolus injection causes changes cation in perioperative and intensive care medicine.