Functional Modules in the Flocculus of the Rabbit Cerebellum
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Sox14 Is Required for a Specific Subset of Cerebello–Olivary Projections
The Journal of Neuroscience, October 31, 2018 • 38(44):9539–9550 • 9539 Development/Plasticity/Repair Sox14 Is Required for a Specific Subset of Cerebello–Olivary Projections Hong-Ting Prekop,1,2 Anna Kroiss,1 Victoria Rook,2 Laskaro Zagoraiou,3,4 Thomas M. Jessell,4 Cathy Fernandes,5 Alessio Delogu,1 and Richard J.T. Wingate2 1Department of Basic and Clinical Neuroscience, Institute of Psychiatry, Psychology and Neuroscience, King’s College London, London, SE5 9NU, United Kingdom, 2MRC Centre for Neurodevelopmental Disorders, Institute of Psychiatry, Psychology and Neuroscience, King’s College London, London SE1 1UL, United Kingdom, 3Biomedical Research Foundation Academy of Athens, 11527, Athens, Greece, 4Department of Neuroscience, Columbia University, New York, 10027, New York, and 5Centre for Social, Genetic and Developmental Psychiatry, Institute of Psychiatry, Psychology and Neuroscience, King’s College London, London, SE5 8AF, United Kingdom We identify Sox14 as an exclusive marker of inhibitory projection neurons in the lateral and interposed, but not the medial, cerebellar nuclei. Sox14؉ neurons make up ϳ80% of Gad1؉ neurons in these nuclei and are indistinguishable by soma size from other inhibitory -neurons. All Sox14؉ neurons of the lateral and interposed cerebellar nuclei are generated at approximately E10/10.5 and extend long ”range, predominantly contralateral projections to the inferior olive. A small Sox14؉ population in the adjacent vestibular nucleus “Y -sends an ipsilateral projection to the oculomotor nucleus. Cerebellar -

The Cerebellum in Sagittal Plane-Anatomic-MR Correlation: 2
667 The Cerebellum in Sagittal Plane-Anatomic-MR Correlation: 2. The Cerebellar Hemispheres Gary A. Press 1 Thin (5-mm) sagittal high-field (1 .5-T) MR images of the cerebellar hemispheres James Murakami2 display (1) the superior, middle, and inferior cerebellar peduncles; (2) the primary white Eric Courchesne2 matter branches to the hemispheric lobules including the central, anterior, and posterior Dean P. Berthoty1 quadrangular, superior and inferior semilunar, gracile, biventer, tonsil, and flocculus; Marjorie Grafe3 and (3) several finer secondary white-matter branches to individual folia within the lobules. Surface features of the hemispheres including the deeper fissures (e.g., hori Clayton A. Wiley3 1 zontal, posterolateral, inferior posterior, and inferior anterior) and shallower sulci are John R. Hesselink best delineated on T1-weighted (short TRfshort TE) and T2-weighted (long TR/Iong TE) sequences, which provide greatest contrast between CSF and parenchyma. Correlation of MR studies of three brain specimens and 11 normal volunteers with microtome sections of the anatomic specimens provides criteria for identifying confidently these structures on routine clinical MR. MR should be useful in identifying, localizing, and quantifying cerebellar disease in patients with clinical deficits. The major anatomic structures of the cerebellar vermis are described in a companion article [1). This communication discusses the topographic relationships of the cerebellar hemispheres as seen in the sagittal plane and correlates microtome sections with MR images. Materials, Subjects, and Methods The preparation of the anatomic specimens, MR equipment, specimen and normal volunteer scanning protocols, methods of identifying specific anatomic structures, and system of This article appears in the JulyI August 1989 issue of AJNR and the October 1989 issue of anatomic nomenclature are described in our companion article [1]. -

Basal Ganglia & Cerebellum
1/2/2019 This power point is made available as an educational resource or study aid for your use only. This presentation may not be duplicated for others and should not be redistributed or posted anywhere on the internet or on any personal websites. Your use of this resource is with the acknowledgment and acceptance of those restrictions. Basal Ganglia & Cerebellum – a quick overview MHD-Neuroanatomy – Neuroscience Block Gregory Gruener, MD, MBA, MHPE Vice Dean for Education, SSOM Professor, Department of Neurology LUHS a member of Trinity Health Outcomes you want to accomplish Basal ganglia review Define and identify the major divisions of the basal ganglia List the major basal ganglia functional loops and roles List the components of the basal ganglia functional “circuitry” and associated neurotransmitters Describe the direct and indirect motor pathways and relevance/role of the substantia nigra compacta 1 1/2/2019 Basal Ganglia Terminology Striatum Caudate nucleus Nucleus accumbens Putamen Globus pallidus (pallidum) internal segment (GPi) external segment (GPe) Subthalamic nucleus Substantia nigra compact part (SNc) reticular part (SNr) Basal ganglia “circuitry” • BG have no major outputs to LMNs – Influence LMNs via the cerebral cortex • Input to striatum from cortex is excitatory – Glutamate is the neurotransmitter • Principal output from BG is via GPi + SNr – Output to thalamus, GABA is the neurotransmitter • Thalamocortical projections are excitatory – Concerned with motor “intention” • Balance of excitatory & inhibitory inputs to striatum, determine whether thalamus is suppressed BG circuits are parallel loops • Motor loop – Concerned with learned movements • Cognitive loop – Concerned with motor “intention” • Limbic loop – Emotional aspects of movements • Oculomotor loop – Concerned with voluntary saccades (fast eye-movements) 2 1/2/2019 Basal ganglia “circuitry” Cortex Striatum Thalamus GPi + SNr Nolte. -

Occurrence of Long-Term Depression in the Cerebellar Flocculus During Adaptation of Optokinetic Response Takuma Inoshita, Tomoo Hirano*
SHORT REPORT Occurrence of long-term depression in the cerebellar flocculus during adaptation of optokinetic response Takuma Inoshita, Tomoo Hirano* Department of Biophysics, Graduate School of Science, Kyoto University, Sakyo-ku, Japan Abstract Long-term depression (LTD) at parallel fiber (PF) to Purkinje cell (PC) synapses has been considered as a main cellular mechanism for motor learning. However, the necessity of LTD for motor learning was challenged by demonstration of normal motor learning in the LTD-defective animals. Here, we addressed possible involvement of LTD in motor learning by examining whether LTD occurs during motor learning in the wild-type mice. As a model of motor learning, adaptation of optokinetic response (OKR) was used. OKR is a type of reflex eye movement to suppress blur of visual image during animal motion. OKR shows adaptive change during continuous optokinetic stimulation, which is regulated by the cerebellar flocculus. After OKR adaptation, amplitudes of quantal excitatory postsynaptic currents at PF-PC synapses were decreased, and induction of LTD was suppressed in the flocculus. These results suggest that LTD occurs at PF-PC synapses during OKR adaptation. DOI: https://doi.org/10.7554/eLife.36209.001 Introduction The cerebellum plays a critical role in motor learning, and a type of synaptic plasticity long-term *For correspondence: depression (LTD) at parallel fiber (PF) to Purkinje cell (PC) synapses has been considered as a primary [email protected]. cellular mechanism for motor learning (Ito, 1989; Hirano, 2013). However, the hypothesis that LTD ac.jp is indispensable for motor learning was challenged by demonstration of normal motor learning in Competing interests: The rats in which LTD was suppressed pharmacologically or in the LTD-deficient transgenic mice authors declare that no (Welsh et al., 2005; Schonewille et al., 2011). -

FIRST PROOF Cerebellum
Article Number : EONS : 0736 GROSS ANATOMY Cerebellum Cortex The cerebellar cortex is an extensive three-layered sheet with a surface approximately 15 cm laterally THE HUMAN CEREBELLUM (‘‘little brain’’) is a and 180 cm rostrocaudally but densely folded around significant part of the central nervous system both three pairs of nuclei. The cortex is divided into three in size and in neural structure. It occupies approxi- transverse lobes: Anterior and posterior lobes are mately one-tenth of the cranial cavity, sitting astride separated by the primary fissure, and the smaller the brainstem, beneath the occipital cortex, and flocculonodular lobe is separated by the poster- contains more neurons than the whole of the cerebral olateral fissure (Fig. 1). The anterior and posterior cortex. It consists of an extensive cortical sheet, lobes are folded into a number of lobules and further densely folded around three pairs of nuclei. The folded into a series of folia. This transverse organiza- cortex contains only five main neural cell types and is tion is then divided at right angles into broad one of the most regular and uniform structures in the longitudinal regions. The central vermis, named for central nervous system (CNS), with an orthogonal its worm-like appearance, is most obvious in the ‘‘crystalline’’ organization. Major connections are posterior lobe. On either side is the paravermal or made to and from the spinal cord, brainstem, and intermediate cortex, which merges into the lateral sensorimotor areas of the cerebral cortex. hemispheres. The most common causes of damage to the cerebellum are stroke, tumors, or multiple sclerosis. -

The Word “Cerebellum” Means: “The Small Brain” . Note That the Cere
Unit VIII – Problem 5 – Physiology: Cerebellum - The word “cerebellum” means: “the small brain”. Note that the cerebellum is not completely separated into 2 hemispheres (they are not clearly demarcated) → the vermis is connecting both cerebellar hemispheres. - Cerebellum contains a very huge number of granular cells (more than all neurons of the central nervous system!) → therefore, you will notice that the grey matter of the cerebellum is bigger than that of the cerebrum. - The motor system: Remember from the previous lectures that the pyramidal tract from the cortex will descend to terminate either in: The lateral part of the ventral horn of the spinal cord: to control fine movements (such as movements of the hands). Or the medial part of the ventral horn of the spinal cord: to control axial muscles (aiding in maintenance of posture). - Also from previous lectures, remember that the idea of movement is generated in the pre- frontal cortex and then it will travel to the pre-motor area which has a lot of programs for the same movement → these programs will be sent to basal ganglia so it can chose only one of them and return it back to the pre-motor cortex (area 6) → then to cerebellum → and eventually to primary motor cortex (area 4). - Cerebellar components: Cerebellar cortex: Vestibulocerebellum. Spinocerebellum. Cerebrocerebellum. Deep cerebellar nuclei: Fastigial nucleus. Interposed nucleus. Dentate nucleus. Cerebellar peduncles: Superior peduncle: connecting it with the midbrain. This peduncle contains efferent pathways mostly to the motor & pre-motor cortices and superior colliculus. Middle peduncle: connecting it with the pons. This peduncle contains afferent fibers from contralateral pons (cortico-ponto-cerebellar fibers). -

…Going One Step Further
…going one step further C20 (1017868) 2 Latin A Encephalon Mesencephalon B Telencephalon 31 Lamina tecti B1 Lobus frontalis 32 Tegmentum mesencephali B2 Lobus temporalis 33 Crus cerebri C Diencephalon 34 Aqueductus mesencephali D Mesencephalon E Metencephalon Metencephalon E1 Cerebellum 35 Cerebellum F Myelencephalon a Vermis G Circulus arteriosus cerebri (Willisii) b Tonsilla c Flocculus Telencephalon d Arbor vitae 1 Lobus frontalis e Ventriculus quartus 2 Lobus parietalis 36 Pons 3 Lobus occipitalis f Pedunculus cerebellaris superior 4 Lobus temporalis g Pedunculus cerebellaris medius 5 Sulcus centralis h Pedunculus cerebellaris inferior 6 Gyrus precentralis 7 Gyrus postcentralis Myelencephalon 8 Bulbus olfactorius 37 Medulla oblongata 9 Commissura anterior 38 Oliva 10 Corpus callosum 39 Pyramis a Genu 40 N. cervicalis I. (C1) b Truncus ® c Splenium Nervi craniales d Rostrum I N. olfactorius 11 Septum pellucidum II N. opticus 12 Fornix III N. oculomotorius 13 Commissura posterior IV N. trochlearis 14 Insula V N. trigeminus 15 Capsula interna VI N. abducens 16 Ventriculus lateralis VII N. facialis e Cornu frontale VIII N. vestibulocochlearis f Pars centralis IX N. glossopharyngeus g Cornu occipitale X N. vagus h Cornu temporale XI N. accessorius 17 V. thalamostriata XII N. hypoglossus 18 Hippocampus Circulus arteriosus cerebri (Willisii) Diencephalon 1 A. cerebri anterior 19 Thalamus 2 A. communicans anterior 20 Sulcus hypothalamicus 3 A. carotis interna 21 Hypothalamus 4 A. cerebri media 22 Adhesio interthalamica 5 A. communicans posterior 23 Glandula pinealis 6 A. cerebri posterior 24 Corpus mammillare sinistrum 7 A. superior cerebelli 25 Hypophysis 8 A. basilaris 26 Ventriculus tertius 9 Aa. pontis 10 A. -

Rubrocerebellar Feedback Loop Isolates the Interposed Nucleus As an Independent Processor of Corollary Discharge Information in Mice
The Journal of Neuroscience, October 18, 2017 • 37(42):10085–10096 • 10085 Systems/Circuits Rubrocerebellar Feedback Loop Isolates the Interposed Nucleus as an Independent Processor of Corollary Discharge Information in Mice Christy S. Beitzel,1 Brenda D. Houck,2 Samantha M. Lewis,2 and Abigail L. Person2 1Neuroscience Graduate Program, and 2Department of Physiology and Biophysics, University of Colorado School of Medicine, Aurora, Colorado 80045 Understanding cerebellar contributions to motor coordination requires deeper insight into how the output structures of the cerebellum, the cerebellar nuclei, integrate their inputs and influence downstream motor pathways. The magnocellular red nucleus (RNm), a brain- stempremotorstructure,isamajortargetoftheinterposednucleus(IN),andhasalsobeendescribedinpreviousstudiestosendfeedback collaterals to the cerebellum. Because such a pathway is in a key position to provide motor efferent information to the cerebellum, satisfying predictions about the use of corollary discharge in cerebellar computations, we studied it in mice of both sexes. Using antero- grade viral tracing, we show that innervation of cerebellum by rubrospinal neuron collaterals is remarkably selective for the IN compared with the cerebellar cortex. Optogenetic activation of the pathway in acute mouse brain slices drove IN activity despite small amplitude synaptic currents, suggesting an active role in IN information processing. Monosynaptic transsynaptic rabies tracing indicated the pathway contacts multiple cell types within the IN. By contrast, IN inputs to the RNm targeted a region that lacked inhibitory neurons. Optogenetic drive of IN inputs to the RNm revealed strong, direct excitation but no inhibition of RNm neurons. Together, these data indicate that the cerebellar nuclei are under afferent control independent of the cerebellar cortex, potentially diversifying its roles in motor control. -
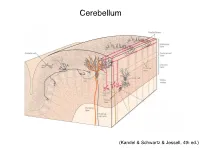
Cerebellum and Activation of the Cerebellum IO ST During Nonmotor Tasks
Cerebellum (Kandel & Schwartz & Jessell, 4th ed.) Granule186 cells are irreducibly smallChapter (6-8 7 μm) stellate cell basket cell outer synaptic layer PC rs cap PC layer grc Go inner 6 μm synaptic layer Figure 7.15 Largest cerebellar neuron occupies more than a 1,000-fold greater volume than smallest neuron. Thin section (~1 μ m) through monkey cerebellar cortex. Purkinje cell body (PC) and nucleus are far larger than those of granule cell (grc). The latter cluster to leave space for mossy fiber terminals to form glomeruli with grc dendritic claws and space for Golgi cells (Go). Note rich network of capillaries (cap). Fine, scat- tered dots are mitochondria. Courtesy of E. Mugnaini. brain ’ s most numerous neuron, this small cost grows large (see also chapter 13). Much of the inner synaptic layer is occupied by the large axon terminals of mossy fibers (figures 7.1 and 7.16). A terminal interlaces with multiple (~15) dendritic claws, each from a different but neighboring granule cell, and forms a complex knot (glomerulus ), nearly as large as a granule cell body (figures 7.1 and 7.16). The mossy fiber axon fires at an unusually high mean rate (up to 200 Hz) and is therefore among the brain’ s thickest (figure 4.6). To match the axon’ s high rate, a terminal expresses 150 active zones, 10 per postsynaptic granule cell (figure 7.16). These sites are capable of driving … and most expensive. 190 Chapter 7 10 4 8 3 9 19 10 10 × 6 × 2 2 4 ATP/s/cell ATP/s/cell ATP/s/m 1 2 0 0 astrocyte astrocyte Golgi cell Golgi cell basket cell basket cell stellate cell stellate cell granule cell granule cell mossy fiber mossy fiber Purkinje cell Purkinje Purkinje cell Purkinje Bergman glia Bergman glia climbing fiber climbing fiber Figure 7.18 Energy costs by cell type. -

Neuroimaging Evidence Implicating Cerebellum in Support of Sensory Cognitive Processes Associated with Thirst
Neuroimaging evidence implicating cerebellum in support of sensory͞cognitive processes associated with thirst Lawrence M. Parsons*†, Derek Denton‡, Gary Egan‡, Michael McKinley‡, Robert Shade‡, Jack Lancaster*, and Peter T. Fox* *Research Imaging Center, Medical School, University of Texas Health Science Center at San Antonio, Floyd Curl Drive, San Antonio, TX 78284; ‡Howard Florey Institute of Experimental Physiology and Medicine, University of Melbourne, Parkville, Victoria 3052, Australia; and §Southwest Foundation for Biomedical Research, P.O. Box 760549, San Antonio, TX 78245-0549 Contributed by Derek A. Denton, December 13, 1999 Recent studies implicate the cerebellum, long considered strictly a and tactile discrimination, kinesthetic sensation, and working motor control structure, in cognitive, sensory, and affective phe- memory, among other processes (6–12). It has been proposed nomenon. The cerebellum, a phylogenetically ancient structure, that the lateral cerebellum may be activated during several has reciprocal ancient connections to the hypothalamus, a struc- motor, perceptual, and cognitive processes specifically because ture important in vegetative functions. The present study investi- of the requirement to monitor and adjust the acquisition of gated whether the cerebellum was involved in vegetative func- sensory data (2, 13). Furthermore, there are reports suggesting tions and the primal emotions engendered by them. Using positron the involvement of posterior vermal cerebellum in affect (14, 15). emission tomography, we examined the effects on the cerebellum The findings implicating the cerebellum in sensory processing of the rise of plasma sodium concentration and the emergence of and emotional states make it of great interest to examine thirst in 10 healthy adults. The correlation of regional cerebral whether the cerebellum has a role in basic vegetative functions blood flow with subjects’ ratings of thirst showed major activation and the primal emotions thus generated, particularly given the in the vermal central lobule. -

On the Function of the Floccular Complex of the Vertebrate Cerebellum: Implications in Paleoneuroanatomy
On the function of the floccular complex of the vertebrate cerebellum: implications in paleoneuroanatomy Sérgio Filipe Ferreira Cardoso Dissertação para obtenção do Grau de Mestre em Paleontologia Orientador: Doutor Rui Alexandre Ferreira Castanhinha Co-orientadores: Doutor Ricardo Miguel Nóbrega Araújo Prof. Doutor Miguel Telles Antunes On the function of the floccular complex of the vertebrate cerebellum: implications in paleoneuroanatomy Sérgio Filipe Ferreira Cardoso Dissertação para obtenção do Grau de Mestre em Paleontologia Orientador: Doutor Rui Alexandre Ferreira Castanhinha Co-orientadores: Doutor Ricardo Miguel Nóbrega Araújo Prof. Doutor Miguel Telles Antunes Successfully defended on 18th November 2015 at FCT-UNL Campus, Portugal, before a juri presided over by: Doutor Paulo Alexandre Rodrigues Roque Legoinha and consisting of: Doutor Gabriel José Gonçalves Martins Doutor Rui Alexandre Ferreira Castanhinha I II Direitos de autor - Copyright Os direitos de autor deste documento pertencem a Sérgio Filipe Ferreira Cardoso, à FCT/UNL, à UNL e à UÉ. A Faculdade de Ciências e Tecnologia, a Universidade Nova de Lisboa e a Universidade de Évora têm o direito, perpétuo e sem limites geográficos, de arquivar e publicar esta dissertação através de exemplares impressos reproduzidos em papel ou de forma digital, ou por qualquer outro meio conhecido ou que venha a ser inventado, e de a divulgar através de repositórios científicos e de admitir a sua cópia e distribuição com objectivos educacionais ou de investigação, não comerciais, desde que seja dado crédito ao autor e editor. Two peer-reviewed abstracts, resulting from this study, were accepted for oral communications (Appendix II). Ferreira-Cardoso, S., Araújo, R., Castanhinha, R., Walsh, S., Martins, R.M.S., Martins, G.G. -

Hagains Uta 2502D 11788.Pdf (6.385Mb)
THE ANTINOCICEPTIVE ROLE OF THE ANTERIOR INTERPOSED NUCLEUS OF THE CEREBELLUM by CHRISTOPHER E. HAGAINS Presented to the Faculty of the Graduate School of The University of Texas at Arlington in Partial Fulfillment of the Requirements for the Degree of DOCTOR OF PHILOSOPHY THE UNIVERSITY OF TEXAS AT ARLINGTON August 2012 Copyright © by Christopher Hagains 2012 All Rights Reserved ACKNOWLEDGEMENTS My education at UTA has been a great, sometimes challenging, experience. Dr. Peng has played a huge role in making it so favorable. He has been very supportive and has helped me exceed my expectations. We’ve had numerous discussions that have challenged my philosophy and helped develop the way I approach science. I will miss Friday lab meetings that consist of boundless brain storming and ideas for tapping into the unknown. I could not have asked for a better mentor. Thanks Dr. Peng! I would also like to thank Dr. Fuchs. He has continuously been a source of simple but difficult questions throughout my time at UTA that always seem to catch me off-guard. He too has been very supportive and always made his resources and knowledge available. Dr. Perrotti has also recently been a great help. She held me accountable to overcoming my writing struggles and helped mentor me through completing my Major Area Paper, and now she has been a strong source of support ensuring the completion of my dissertation. Thanks also to my dissertation committee. I appreciate the time and effort it takes to make good scientists. Each of you has provided a unique element that has helped me become a sharper philosopher and scientist.