Persistence of Host Defence Behaviour in the Absence of Avian Brood
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Natural History of Japanese Birds
Natural History of Japanese Birds Hiroyoshi Higuchi English text translated by Reiko Kurosawa HEIBONSHA 1 Copyright © 2014 by Hiroyoshi Higuchi, Reiko Kurosawa Typeset and designed by: Washisu Design Office Printed in Japan Heibonsha Limited, Publishers 3-29 Kanda Jimbocho, Chiyoda-ku Tokyo 101-0051 Japan All rights reserved. No part of this publication may be reproduced or transmitted in any form or by any means without permission in writing from the publisher. The English text can be downloaded from the following website for free. http://www.heibonsha.co.jp/ 2 CONTENTS Chapter 1 The natural environment and birds of Japan 6 Chapter 2 Representative birds of Japan 11 Chapter 3 Abundant varieties of forest birds and water birds 13 Chapter 4 Four seasons of the satoyama 17 Chapter 5 Active life of urban birds 20 Chapter 6 Interesting ecological behavior of birds 24 Chapter 7 Bird migration — from where to where 28 Chapter 8 The present state of Japanese birds and their future 34 3 Natural History of Japanese Birds Preface [BOOK p.3] Japan is a beautiful country. The hills and dales are covered “satoyama”. When horsetail shoots come out and violets and with rich forest green, the river waters run clear and the moun- cherry blossoms bloom in spring, birds begin to sing and get tain ranges in the distance look hazy purple, which perfectly ready for reproduction. Summer visitors also start arriving in fits a Japanese expression of “Sanshi-suimei (purple mountains Japan one after another from the tropical regions to brighten and clear waters)”, describing great natural beauty. -

GRUNDSTEN Japan 0102 2016
Birding Japan (M. Grundsten, Sweden) 2016 Japan, January 30th - February 14th 2016 Karuizawa – E Hokkaido – S Kyushu – Okinawa – Hachijo-jima Front cover Harlequin Duck Histrionicus histrionicus, common along eastern Hokkaido coasts. Photo: Måns Grundsten Participants Måns Grundsten ([email protected], compiler, most photos), Mattias Andersson, Mattias Gerdin, Sweden. Highlights • A shy Solitary Snipe in the main stream at Karuizawa. • Huge-billed Japanese Grosbeaks and a neat 'griseiventris' Eurasian Bullfinch at Karuizawa. • A single Rustic Bunting behind 7/Eleven at Karuizawa. • Amazing auks from the Oarai-Tomakomai ferry. Impressive numbers of Rhinoceros Auklet! • Parakeet Auklet fly-bys. • Blakiston's Fish Owl in orderly fashion at Rausu. • Displaying Black Scoters at Notsuke peninsula. • Majestic Steller's Sea Eagles in hundreds. • Winter gulls at Hokkaido. • Finding a vagrant Golden-crowned Sparrow at Kiritappu at the same feeders as Asian Rosy Finches. • No less than 48(!) Rock Sandpipers. • A lone immature Red-faced Cormorants on cliffs at Cape Nosappu. • A pair of Ural Owls on day roost at Kushiro. • Feeding Ryukyu Minivets at Lake Mi-ike. • Fifteen thousand plus cranes at Arasaki. • Unexpectedly productive Kogawa Dam – Long-billed Plover. • Saunders's Gulls at Yatsushiro. • Kin Ricefields on Okinawa, easy birding, lots of birds, odd-placed Tundra Bean Geese. • Okinawa Woodpecker and Rail within an hour close to Fushigawa Dam, Yanbaru. • Whistling Green Pigeon eating fruits in Ada Village. • Vocal Ryukyu Robins. • Good shorebird diversity in Naha. • Male Izu Thrush during a short break on Hachijo-jima. • Triple Albatrosses! • Bulwer's Petrel close to the ship. Planning the trip – Future aspects When planning a birding trip to Japan there is a lot of consideration to be made. -

Winter Bird Highlights 2013
FROM PROJECT FEEDERWAtch 2012–13 Focus on citizen science • Volume 9 Winter BirdHighlights Winter npredictability is one constant as each winter Focus on Citizen Science is a publication highlight- ing the contributions of citizen scientists. This is- brings surprises to our feeders. The 2012–13 sue, Winter Bird Highlights 2013, is brought to you by Project FeederWatch, a research and education proj- season broke many regional records with sis- ect of the Cornell Lab of Ornithology and Bird Studies U Canada. Project FeederWatch is made possible by the kins and nuthatches moving south in record numbers efforts and support of thousands of citizen scientists. to tantalize FeederWatchers across much of the con- Project FeederWatch Staff tinent. This remarkable year also brought a record- David Bonter breaking number of FeederWatchers, with more than Project Leader, USA Janis Dickinson 20,000 participants in the US and Canada combined! Director of Citizen Science, USA Kristine Dobney Whether you’ve been FeederWatching for 26 years or Project Assistant, Canada Wesley Hochachka this is your first season counting, the usual suspects— Senior Research Associate, USA chickadees, juncos, and woodpeckers—always bring Anne Marie Johnson Project Assistant, USA familiarity and enjoyment, as well as valuable data, Rosie Kirton Project Support, Canada even if you don’t observe anything unusual. Whichever Denis Lepage birds arrive at your feeder, we hope they will bring a Senior Scientist, Canada Susan E. Newman sense of wonder that captures your attention. Thanks Project Assistant, USA for sharing your observations and insights with us and, Kerrie Wilcox Project Leader, Canada most importantly, Happy FeederWatching. -

Pinecrest Golf Course
Pinecrest Golf Course Birds on the Course In North America the Red-tailed Hawk is one of three species colloquially known as the “chicken hawk” or "hen hawk" even though chickens are not a major part of their diet. They were given this name in earlier times, when free-ranging chickens were preyed upon by first-year juveniles. They are also called buzzard hawks or red hawks. Red-tailed Hawks are easily recognized by their brick-red colored tails, from which its common name was derived. In the wild, they are expected to live for 10 -21 years. They reach reproductive maturity when they are about 3 years old. The Red-tailed Hawks is a bird of prey found in North and Central America, and in the West Indies. Throughout their range, they typically live in forests near open country or - depending on their range - in swamps, taigas and deserts. This species is legally protected in Canada, Mexico and the United States by the international Migratory Bird Treaty Act. In the United States, they are also protected by state, provincial and federal bird protection laws, making it illegal to keep hawks (without a permit) in captivity, or to Red-Tailed Hawk hunt them; disturb nests or eggs; even collecting their feathers is against the law. Red-Shouldered Hawk The Red-shouldered Sharp- Hawk is a medium-sized Hawk. A common Shinned forest-dwelling hawk of the East and California, Hawk the Red-shouldered Hawk favors woodlands near water. It is perhaps The sharp-shinned hawk is small with blue-gray upper parts and rufous bars on white the most vocal under parts. -

A Birder's Guide to Cook County, Northeastern Minnesota Birding
A Birder’s Guide to Cook County, Northeastern Minnesota This guide will help you find the birds of Cook County, one of the best birding areas in the upper midwest. The shore of Lake Superior and the wildlands of the northeast are natural treasures that are especially rich in birds. Descriptions of the locatoins can be found inside, along with information about how to make the most of your birding during each season of the year. Birding around the year Spring: The migration is always most exciting along the shore of Lake Superior. Spring migration is smaller than fall, but spring specialties include Tundra Swan, Sandhill Crane, Gray-cheeked Thrush, American Tree Sparrow, Harris’ Sparrow, Lapland Longspur and Rusty Blackbird. Boreal species like Black-backed Woodpecker, Boreal Owl and Northern Saw-whet Owl begin nesting during spring, which can begin as early as March and extend until June. Summer: In summer the excitement moves inland where specialties inclue Common Loon, American Black Duck, Bald Eagle, Ruffled Grouse, American Woodcock, Black-billed Cuckoo, Barred Owl, Northern Saw- whet Owl, Whip-poor-will, Olive-sided, Yellow-bellied, and Alder Flycatchers, Gray Jay, Boreal Chickadee, Winter and Sedge Wrens, 20 species of warblers, Le Conte’s Sparrow, and Evening Grosbeak. The summer breeding season extends from late May through early August. Autumn: the fall migration along the Norht Shore of Lake Superior is not to be missed! Beginning with the sight of thousands of Common Nighthawks in late August, the sheer quantity of birds moving down the shore makes this area a world-class migration route. -
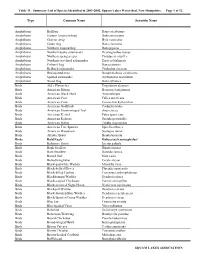
Summary Data
Table 11. Summary List of Species Identified in 2001-2002, Squam Lakes Watershed, New Hampshire. Page 1 of 12 Type Common Name Scientific Name Amphibians Bullfrog Rana catesbeiana Amphibians Eastern American toad Bufo americanus Amphibians Gray treefrog Hyla versicolor Amphibians Green frog Rana clamitans Amphibians Northern leopard frog Rana pipiens Amphibians Northern dusky salamander Desmognathus fuscus Amphibians Northern spring peeper Pseudacris crucifer Amphibians Northern two-lined salamander Eurycea bislineata Amphibians Pickerel frog Rana palustris Amphibians Redback salamander Plethodon cinereus Amphibians Red-spotted newt Notophthalmus viridescens Amphibians Spotted salamander Ambystoma maculatum Amphibians Wood frog Rana sylvatica Birds Alder Flycatcher Empidonax alnorum Birds American Bittern Botaurus lentiginosus Birds American Black Duck Anas rubripes Birds American Coot Fulica americana Birds American Crow Corvus brachyrhynchos Birds American Goldfinch Carduelis tristis Birds American Green-winged Teal Anas crecca Birds American Kestrel Falco sparverius Birds American Redstart Setophaga ruticilla Birds American Robin Turdus migratorius Birds American Tree Sparrow Spizella arborea Birds American Woodcock Scolopax minor Birds Atlantic Brant Branta bernicla Birds Bald Eagle* Haliaeetus leucocephalus* Birds Baltimore Oriole Icterus galbula Birds Bank Swallow Riparia riparia Birds Barn Swallow Hirundo rustica Birds Barred Owl Strix varia Birds Belted Kingfisher Ceryle alcyon Birds Black-and-white Warbler Mniotilta varia Birds -

Winter Bird Highlights 2015, Is Brought to You by U.S
Winter Bird Highlights FROM PROJECT FEEDERWATCH 2014–15 FOCUS ON CITIZEN SCIENCE • VOLUME 11 Focus on Citizen Science is a publication highlight- FeederWatch welcomes new ing the contributions of citizen scientists. This is- sue, Winter Bird Highlights 2015, is brought to you by U.S. project assistant Project FeederWatch, a research and education proj- ect of the Cornell Lab of Ornithology and Bird Studies Canada. Project FeederWatch is made possible by the e are pleased to have a new efforts and support of thousands of citizen scientists. Wteam member on board! Meet Chelsea Benson, a new as- Project FeederWatch Staff sistant for Project FeederWatch. Chelsea will also be assisting with Cornell Lab of Ornithology NestWatch, another Cornell Lab Janis Dickinson citizen-science project. She will Director of Citizen Science be responding to your emails and Emma Greig phone calls and helping to keep Project Leader and Editor the website and social media pages Anne Marie Johnson Project Assistant up-to-date. Chelsea comes to us with a back- Chelsea Benson Project Assistant ground in environmental educa- Wesley Hochachka tion and conservation. She has worked with schools, community Senior Research Associate organizations, and local governments in her previous positions. Diane Tessaglia-Hymes She incorporated citizen science into her programming and into Design Director regional events like Day in the Life of the Hudson River. Chelsea holds a dual B.A. in psychology and English from Bird Studies Canada Allegheny College and an M.A. in Social Science, Environment Kerrie Wilcox and Community, from Humboldt State University. Project Leader We are excited that Chelsea has brought her energy and en- Rosie Kirton thusiasm to the Cornell Lab, where she will no doubt mobilize Project Support even more people to monitor bird feeders (and bird nests) for Kristine Dobney Project Assistant science. -

Birds of Anchorage Checklist
ACCIDENTAL, CASUAL, UNSUBSTANTIATED KEY THRUSHES J F M A M J J A S O N D n Casual: Occasionally seen, but not every year Northern Wheatear N n Accidental: Only one or two ever seen here Townsend’s Solitaire N X Unsubstantiated: no photographic or sample evidence to support sighting Gray-cheeked Thrush N W Listed on the Audubon Alaska WatchList of declining or threatened species Birds of Swainson’s Thrush N Hermit Thrush N Spring: March 16–May 31, Summer: June 1–July 31, American Robin N Fall: August 1–November 30, Winter: December 1–March 15 Anchorage, Alaska Varied Thrush N W STARLINGS SPRING SUMMER FALL WINTER SPECIES SPECIES SPRING SUMMER FALL WINTER European Starling N CHECKLIST Ross's Goose Vaux's Swift PIPITS Emperor Goose W Anna's Hummingbird The Anchorage area offers a surprising American Pipit N Cinnamon Teal Costa's Hummingbird Tufted Duck Red-breasted Sapsucker WAXWINGS diversity of habitat from tidal mudflats along Steller's Eider W Yellow-bellied Sapsucker Bohemian Waxwing N Common Eider W Willow Flycatcher the coast to alpine habitat in the Chugach BUNTINGS Ruddy Duck Least Flycatcher John Schoen Lapland Longspur Pied-billed Grebe Hammond's Flycatcher Mountains bordering the city. Fork-tailed Storm-Petrel Eastern Kingbird BOHEMIAN WAXWING Snow Bunting N Leach's Storm-Petrel Western Kingbird WARBLERS Pelagic Cormorant Brown Shrike Red-faced Cormorant W Cassin's Vireo Northern Waterthrush N For more information on Alaska bird festivals Orange-crowned Warbler N Great Egret Warbling Vireo Swainson's Hawk Red-eyed Vireo and birding maps for Anchorage, Fairbanks, Yellow Warbler N American Coot Purple Martin and Kodiak, contact Audubon Alaska at Blackpoll Warbler N W Sora Pacific Wren www.AudubonAlaska.org or 907-276-7034. -

Kenai National Wildlife Refuge Species List, Version 2018-07-24
Kenai National Wildlife Refuge Species List, version 2018-07-24 Kenai National Wildlife Refuge biology staff July 24, 2018 2 Cover image: map of 16,213 georeferenced occurrence records included in the checklist. Contents Contents 3 Introduction 5 Purpose............................................................ 5 About the list......................................................... 5 Acknowledgments....................................................... 5 Native species 7 Vertebrates .......................................................... 7 Invertebrates ......................................................... 55 Vascular Plants........................................................ 91 Bryophytes ..........................................................164 Other Plants .........................................................171 Chromista...........................................................171 Fungi .............................................................173 Protozoans ..........................................................186 Non-native species 187 Vertebrates ..........................................................187 Invertebrates .........................................................187 Vascular Plants........................................................190 Extirpated species 207 Vertebrates ..........................................................207 Vascular Plants........................................................207 Change log 211 References 213 Index 215 3 Introduction Purpose to avoid implying -

The Birds of New York State
__ Common Goldeneye RAILS, GALLINULES, __ Baird's Sandpiper __ Black-tailed Gull __ Black-capped Petrel Birds of __ Barrow's Goldeneye AND COOTS __ Little Stint __ Common Gull __ Fea's Petrel __ Smew __ Least Sandpiper __ Short-billed Gull __ Cory's Shearwater New York State __ Clapper Rail __ Hooded Merganser __ White-rumped __ Ring-billed Gull __ Sooty Shearwater __ King Rail © New York State __ Common Merganser __ Virginia Rail Sandpiper __ Western Gull __ Great Shearwater Ornithological __ Red-breasted __ Corn Crake __ Buff-breasted Sandpiper __ California Gull __ Manx Shearwater Association Merganser __ Sora __ Pectoral Sandpiper __ Herring Gull __ Audubon's Shearwater Ruddy Duck __ Semipalmated __ __ Iceland Gull __ Common Gallinule STORKS Sandpiper www.nybirds.org GALLINACEOUS BIRDS __ American Coot __ Lesser Black-backed __ Wood Stork __ Northern Bobwhite __ Purple Gallinule __ Western Sandpiper Gull FRIGATEBIRDS DUCKS, GEESE, SWANS __ Wild Turkey __ Azure Gallinule __ Short-billed Dowitcher __ Slaty-backed Gull __ Magnificent Frigatebird __ Long-billed Dowitcher __ Glaucous Gull __ Black-bellied Whistling- __ Ruffed Grouse __ Yellow Rail BOOBIES AND GANNETS __ American Woodcock Duck __ Spruce Grouse __ Black Rail __ Great Black-backed Gull __ Brown Booby __ Wilson's Snipe __ Fulvous Whistling-Duck __ Willow Ptarmigan CRANES __ Sooty Tern __ Northern Gannet __ Greater Prairie-Chicken __ Spotted Sandpiper __ Bridled Tern __ Snow Goose __ Sandhill Crane ANHINGAS __ Solitary Sandpiper __ Least Tern __ Ross’s Goose __ Gray Partridge -

(2007): Birds of the Aleutian Islands, Alaska Please
Bold* = Breeding Sp Su Fa Wi Bold* = Breeding Sp Su Fa Wi OSPREYS FINCHES Osprey Ca Ca Ac Brambling I Ca Ca EAGLES and HAWKS Hawfinch I Ca Northern Harrier I I I Common Rosefinch Ca Eurasian Sparrowhawk Ac (Ac) Pine Grosbeak Ca Bald Eagle* C C C C Asian Rosy-Finch Ac Rough-legged Hawk Ac Ca Ca Gray-crowned Rosy-Finch* C C C C OWLS (griseonucha) Snowy Owl I Ca I I Gray-crowned Rosy-Finch (littoralis) Ac Short-eared Owl* R R R U Oriental Greenfinch Ca FALCONS Common Redpoll I Ca I I Eurasian Kestrel Ac Ac Hoary Redpoll Ca Ac Ca Ca Merlin Ca I Red Crossbill Ac Gyrfalcon* R R R R White-winged Crossbill Ac Peregrine Falcon* (pealei) U U C U Pine Siskin I Ac I SHRIKES LONGSPURS and SNOW BUNTINGS Northern Shrike Ca Ca Ca Lapland Longspur* Ac-C C C-Ac Ac CROWS and JAYS Snow Bunting* C C C C Common Raven* C C C C McKay's Bunting Ca Ac LARKS EMBERIZIDS Sky Lark Ca Ac Rustic Bunting Ca Ca SWALLOWS American Tree Sparrow Ac Tree Swallow Ca Ca Ac Savannah Sparrow Ca Ca Ca Bank Swallow Ac Ca Ca Song Sparrow* C C C C Cliff Swallow Ca Golden-crowned Sparrow Ac Ac Barn Swallow Ca Dark-eyed Junco Ac WRENS BLACKBIRDS Pacific Wren* C C C U Rusty Blackbird Ac LEAF WARBLERS WOOD-WARBLERS Bold* = Breeding Sp Su Fa Wi Wood Warbler Ac Yellow Warbler Ac Dusky Warbler Ac Blackpoll Warbler Ac DUCKS, GEESE and SWANS Kamchatka Leaf Warbler Ac Yellow-rumped Warbler Ac Emperor Goose C-I Ca I-C C OLD WORLD FLYCATCHERS "HYPOTHETICAL" species needing more documentation Snow Goose Ac Ac Gray-streaked Flycatcher Ca American Golden-plover (Ac) Greater White-fronted Goose I -

Inner Mongolia Cumulative Bird List Column A
China: Inner Mongolia Cumulative Bird List Column A: total number of days that the species was recorded in 2016 Column B: maximum daily count for that particular species Column C: H = Heard only; (H) = Heard more often than seen Globally threatened species as defined by BirdLife International (2004) Threatened birds of the world 2004 CD-Rom Cambridge, U.K. BirdLife International are identified as follows: EN = Endangered; VU = Vulnerable; NT = Near- threatened. A B C Ruddy Shelduck 2 3 Tadorna ferruginea Mandarin Duck 1 10 Aix galericulata Gadwall 2 12 Anas strepera Falcated Teal 1 4 Anas falcata Eurasian Wigeon 1 2 Anas penelope Mallard 5 40 Anas platyrhynchos Eastern Spot-billed Duck 3 12 Anas zonorhyncha Eurasian Teal 2 12 Anas crecca Baer's Pochard EN 1 4 Aythya baeri Ferruginous Pochard NT 3 49 Aythya nyroca Tufted Duck 1 1 Aythya fuligula Common Goldeneye 2 7 Bucephala clangula Hazel Grouse 4 14 Tetrastes bonasia Daurian Partridge 1 5 Perdix dauurica Brown Eared Pheasant VU 2 15 Crossoptilon mantchuricum Common Pheasant 8 10 Phasianus colchicus Little Grebe 4 60 Tachybaptus ruficollis Great Crested Grebe 3 15 Podiceps cristatus Eurasian Bittern 3 1 Botaurus stellaris Yellow Bittern 1 1 H Ixobrychus sinensis Black-crowned Night Heron 3 2 Nycticorax nycticorax Chinese Pond Heron 1 1 Ardeola bacchus Grey Heron 3 5 Ardea cinerea Great Egret 1 1 Ardea alba Little Egret 2 8 Egretta garzetta Great Cormorant 1 20 Phalacrocorax carbo Western Osprey 2 1 Pandion haliaetus Black-winged Kite 2 1 Elanus caeruleus ________________________________________________________________________________________________________ WINGS ● 1643 N. Alvernon Way Ste.