Baby Gorilla: Photographic and Descriptive Atlas of Skeleton
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

14-Anatomy of Forearm
FOREARM By : Prof.Saeed Abulmakarem. Dr. Sanaa Al-Sharawy OBJECTIVES §At the end of this lecture, the student should able to : §List the names of the Flexors Group of Forearm (superficial & deep muscles). §Identify the common flexor origin of flexor muscles and their innervation & movements. §Identify supination & poronation and list the muscles produced these 2 movements. §List the names of the Extensor Group of Forearm (superficial & deep muscles). §Identify the common extensor origin of extensor musles and their innervation & movements. n The forearm extends from elbow to wrist. n It posses two bones radius laterally & Ulna medially. n The two bones are connected together by the interosseous membrane. n This membrane allows movement of Pronation and Supination while the two bones are connected together. n Also it gives origin for the deep muscles. § The forearm is Fascial Compartments of the Forearm enclosed in a sheath of deep fascia, which is attached to the posterior border of the ulna . §This fascial sheath, together with the interosseous membrane & fibrous intermuscular septa, divides the forearm into compartments, each having its own muscles, nerves, and blood supply. These muscles: 8 FLEXOR GROUP § Act on the elbow & wrist joints and those of the fingers. § Form fleshy masses in the proximal part and become tendinous in the distal part of the forearm. •Arranged in three groups: I-Superficial: 4 Ø Pronator teres Ø Flexor carpi radialis Ø Palmaris longus III- Deep: 3 Ø Flexor carpi ulnaris Ø Flexor digitorum profundus II-Intermediate: 1 Ø Flexor pollicis longus Ø Ø Flexor digitorum superficialis Pronator quadratus n Superficial Flexors: n They arise - more or less- from the common flexor origin (front of medial epicondyle). -

Compiled for Lower Limb
Updated: December, 9th, 2020 MSI ANATOMY LAB: STRUCTURE LIST Lower Extremity Lower Extremity Osteology Hip bone Tibia • Greater sciatic notch • Medial condyle • Lesser sciatic notch • Lateral condyle • Obturator foramen • Tibial plateau • Acetabulum o Medial tibial plateau o Lunate surface o Lateral tibial plateau o Acetabular notch o Intercondylar eminence • Ischiopubic ramus o Anterior intercondylar area o Posterior intercondylar area Pubic bone (pubis) • Pectineal line • Tibial tuberosity • Pubic tubercle • Medial malleolus • Body • Superior pubic ramus Patella • Inferior pubic ramus Fibula Ischium • Head • Body • Neck • Ramus • Lateral malleolus • Ischial tuberosity • Ischial spine Foot • Calcaneus Ilium o Calcaneal tuberosity • Iliac fossa o Sustentaculum tali (talar shelf) • Anterior superior iliac spine • Anterior inferior iliac spine • Talus o Head • Posterior superior iliac spine o Neck • Posterior inferior iliac spine • Arcuate line • Navicular • Iliac crest • Cuboid • Body • Cuneiforms: medial, intermediate, and lateral Femur • Metatarsals 1-5 • Greater trochanter • Phalanges 1-5 • Lesser trochanter o Proximal • Head o Middle • Neck o Distal • Linea aspera • L • Lateral condyle • L • Intercondylar fossa (notch) • L • Medial condyle • L • Lateral epicondyle • L • Medial epicondyle • L • Adductor tubercle • L • L • L • L • 1 Updated: December, 9th, 2020 Lab 3: Anterior and Medial Thigh Anterior Thigh Medial thigh General Structures Muscles • Fascia lata • Adductor longus m. • Anterior compartment • Adductor brevis m. • Medial compartment • Adductor magnus m. • Great saphenous vein o Adductor hiatus • Femoral sheath o Compartments and contents • Pectineus m. o Femoral canal and ring • Gracilis m. Muscles & Associated Tendons Nerves • Tensor fasciae lata • Obturator nerve • Iliotibial tract (band) • Femoral triangle: Boundaries Vessels o Inguinal ligament • Obturator artery o Sartorius m. • Femoral artery o Adductor longus m. -

Clinical Anatomy of the Lower Extremity
Государственное бюджетное образовательное учреждение высшего профессионального образования «Иркутский государственный медицинский университет» Министерства здравоохранения Российской Федерации Department of Operative Surgery and Topographic Anatomy Clinical anatomy of the lower extremity Teaching aid Иркутск ИГМУ 2016 УДК [617.58 + 611.728](075.8) ББК 54.578.4я73. К 49 Recommended by faculty methodological council of medical department of SBEI HE ISMU The Ministry of Health of The Russian Federation as a training manual for independent work of foreign students from medical faculty, faculty of pediatrics, faculty of dentistry, protocol № 01.02.2016. Authors: G.I. Songolov - associate professor, Head of Department of Operative Surgery and Topographic Anatomy, PhD, MD SBEI HE ISMU The Ministry of Health of The Russian Federation. O. P.Galeeva - associate professor of Department of Operative Surgery and Topographic Anatomy, MD, PhD SBEI HE ISMU The Ministry of Health of The Russian Federation. A.A. Yudin - assistant of department of Operative Surgery and Topographic Anatomy SBEI HE ISMU The Ministry of Health of The Russian Federation. S. N. Redkov – assistant of department of Operative Surgery and Topographic Anatomy SBEI HE ISMU THE Ministry of Health of The Russian Federation. Reviewers: E.V. Gvildis - head of department of foreign languages with the course of the Latin and Russian as foreign languages of SBEI HE ISMU The Ministry of Health of The Russian Federation, PhD, L.V. Sorokina - associate Professor of Department of Anesthesiology and Reanimation at ISMU, PhD, MD Songolov G.I K49 Clinical anatomy of lower extremity: teaching aid / Songolov G.I, Galeeva O.P, Redkov S.N, Yudin, A.A.; State budget educational institution of higher education of the Ministry of Health and Social Development of the Russian Federation; "Irkutsk State Medical University" of the Ministry of Health and Social Development of the Russian Federation Irkutsk ISMU, 2016, 45 p. -

Small Muscles of the Hand
By the name of Allah Small muscles of the hand Revision: The palmar aponeurosis is triangular in shape with apex and base. It is divided into 4 bands that radiate to the medial four fingers. Dupuytren’s Contracture: • A localized thickening and shortening of palmar aponeurosis that limits hand function (it is permanent) • Fibrosis pulls the ring finger then the little finger into partial flexion at the MCP joints, followed by flexion of their proximal interphalangeal joints • Usual treatment: Treated by surgical excision of fibrous bands followed by physiotherapy. Alternative treatment: Injection of the enzyme Collagenase into the contracted bands of the fibrous tissue. Keep in mind: • When the muscle Isn’t functioning: It is Relaxed. While it is in action: It is contracted. • Contraction DIFFERS from contracture. Contracture means permanent shortening. 18 th \Mar\2012 1 Small muscles of the hand: Arranged in five groups + 1 muscle, as the following: 1- Thenar muscles: (three in number) each moves the thumb according to its name: • Flexor pollicis brevis: assists the flexor pollicis longus in the flexion of MCP joint of the thumb. • Abductor pollicis brevis: abduction of the thumb. • Opponens pollicis: pulls the thumb medially and forward across the palm (as in counting fingers, shown in the figure below). All supplied by median nerve. 2- Hypothenar muscles: (three in number) each moves the little finger according to its name: • Flexor digiti minimi. • Abductor digit minimi. • Opponens digiti minimi. All supplied by deep branch of ulnar nerve. Only the thumb and little finger got opponens muscles, the Dr said it is because of the long distance between the two fingers ☺ 3- Adductor pollicis muscle: • It has got two heads: horizontal( transverse) and Oblique. -

Extensor Pollicis Longus Superficialis and Extensor Indicis Superficialis, Can They Be Considered As a New Anatomical Variation in the Long Extensors of Fingers?
Int. J. Curr. Res. Med. Sci. (2016). 2(12): 27-32 International Journal of Current Research in Medical Sciences ISSN: 2454-5716 www.ijcrims.com Volume 2, Issue 12 -2016 Case study DOI: http://dx.doi.org/10.22192/ijcrms.2016.02.12.005 Extensor pollicis longus superficialis and extensor indicis superficialis, can they be considered as a new anatomical variation in the long extensors of fingers? Ahmed Farid Al-Neklawy, M.D. Anatomy and Embryology Department, Faculty of Medicine, Ain Shams University, Cairo, Egypt E-mail: [email protected], Tel: 00201001850336 Running title: Two extra muscles for thumb and index fingers Abstract Background: Variations of anomalies of hand extensors have been described by many authors. These anomalies are often discovered during routine surgical procedures and cadaveric dissections. Being aware of such anomalies is important to the physician in order to avoid unintentional damage to healthy tendons during surgical procedures. In addition, accessory tendons have the potential to be used in the surgical repair or replacement of damaged tendons. We reported a cadaveric case with bilateral two additional superficial extensors to the thumb and index fingers with unique features. The names of extensor pollicis longus superficialis (EPL-S) and extensor indicis superficialis(EI-S) were proposed. Methods: A female cadaver was used in this study. Bilateral dissection of the forearm and wrist was done. Results: Two extra muscles were observed in the superficial group of the extensors of the forearm. They were situated between extensor carpi radialis brevis and extensor digitorum muscles. Both muscles originated from the common extensor origin. -

Section 1 Upper Limb Anatomy 1) with Regard to the Pectoral Girdle
Section 1 Upper Limb Anatomy 1) With regard to the pectoral girdle: a) contains three joints, the sternoclavicular, the acromioclavicular and the glenohumeral b) serratus anterior, the rhomboids and subclavius attach the scapula to the axial skeleton c) pectoralis major and deltoid are the only muscular attachments between the clavicle and the upper limb d) teres major provides attachment between the axial skeleton and the girdle 2) Choose the odd muscle out as regards insertion/origin: a) supraspinatus b) subscapularis c) biceps d) teres minor e) deltoid 3) Which muscle does not insert in or next to the intertubecular groove of the upper humerus? a) pectoralis major b) pectoralis minor c) latissimus dorsi d) teres major 4) Identify the incorrect pairing for testing muscles: a) latissimus dorsi – abduct to 60° and adduct against resistance b) trapezius – shrug shoulders against resistance c) rhomboids – place hands on hips and draw elbows back and scapulae together d) serratus anterior – push with arms outstretched against a wall 5) Identify the incorrect innervation: a) subclavius – own nerve from the brachial plexus b) serratus anterior – long thoracic nerve c) clavicular head of pectoralis major – medial pectoral nerve d) latissimus dorsi – dorsal scapular nerve e) trapezius – accessory nerve 6) Which muscle does not extend from the posterior surface of the scapula to the greater tubercle of the humerus? a) teres major b) infraspinatus c) supraspinatus d) teres minor 7) With regard to action, which muscle is the odd one out? a) teres -
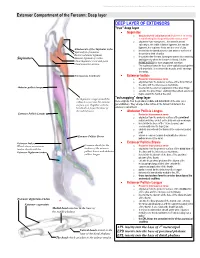
Extensor Compartment of the Forearm: Deep Layer
This document was created by Alex Yartsev ([email protected]); if I have used your data or images and forgot to reference you, please email me. Extensor Compartment of the Forearm: Deep layer DEEP LAYER OF EXTENSORS "true" deep layer Supinator o deep branch of radial nerve which pierces it on its way to transforming into the posterior interosseous nerve o originates from everywhere... the lateral humeral epicondyle, the radial collateral ligament, the annular ligament, the supinator fossa and the crest of ulna Attachments of the Supinator to the o inserts into the lateral posterior and anterior surfaces of Epicondyle of humerus the proximal third of radius Radial collateral ligament o it supinates the forearm, turning the arm to face anteriorly Annular ligament of radius Supinator and superiorly when the forearm is flexed. It is the Ulnar Supinator crest and fossa PRIME MOVER for slow unopposed suination Ulnar posterior surface o The supinator forms the floor of the cubital fossa together with brachialis. It is a sheet-like muscle, and it envelops the radius. Interosseous membrane Extensor Indicis o Posterior interosseous nerve o originates from the posterior surface of the distal third of the ulna, and the interosseous membrane Abductor pollicis longus o inserts into the extensor expansion of the index finger o extends the index finger, enabling independent extension o helps extend the hand at the wrist "outcropping" deep layer the Supinator wraps around the radius to insert into the anterior these originate from the proximal, middle and distal thirds of the ulna (as a surface of it. -

Über Dendrolagus Dorianus. Albertina Carlsson
ZOBODAT - www.zobodat.at Zoologisch-Botanische Datenbank/Zoological-Botanical Database Digitale Literatur/Digital Literature Zeitschrift/Journal: Zoologische Jahrbücher. Abteilung für Systematik, Geographie und Biologie der Tiere Jahr/Year: 1914 Band/Volume: 36 Autor(en)/Author(s): Carlsson Albertina Artikel/Article: Über Dendrolagus dorianus. 547-617 © Biodiversity Heritage Library, http://www.biodiversitylibrary.org/; www.zobodat.at Nachdruck verboten. Übersetzungsrecht vorbehalten. Über Dendrolagus dorianus. Von Albertina Carlsson. (Aus dem Zootomischen Institut der Universität zu Stockholm.) Mit Tafel 20-22. Nach HuxLEY (29), Dollo (18) und Bensley (6) waren die Beuteltiere ursprünglich Baumtiere ; verschiedene aber haben sich später einem Leben auf dem Boden angepaßt, einige vor dem Auftreten des Syndactylismus, womit eine Reduktion des opponierbaren Hallux zusammenhängt, andere nach dem Auftreten desselben ; letztere Fuß- form repräsentiert eine vollständigere Adaption an die arboricole Lebens- weise (19, p. 168). Neuerdings iiat Matthew darzulegen versucht (37), daß alle bekannten Mammalia, von den Prototheria abgesehen, von Tieren abstammen, welche auf Bäumen gelebt haben; also kann die von Dollo und Bensley ausgesprochene Behauptung auch auf die Monodelphia ausgedehnt w^erden. Unter den dem terrestrischen Leben besonders angepaßten Macro- podidae gibt es eine Gattung, Dendrolagus, die im Äußeren deutlich mit den Känguruhs übereinstimmt, die aber durch geringere Ver- schiedenheit in der Länge der vorderen und der hinteren Extremität von denselben abweicht. Teils hüpft das Tier auf dem Boden, wobei es denselben mit den Vorderbeinen berührt oder bei langsamem Hüpfen nur die eine Hand auf den Boden setzt und die andere erhoben hält (1, p. 409), teils klettert es auf den Bäumen, wobei der Stamm mit den Vorderfüßen umfaßt wird (8, p. -

Proximal Versus Distal Continuous Adductor Canal Blocks
Proximal Versus Distal Continuous Adductor Canal Blocks: Does Varying Perineural Catheter Location Influence Analgesia? A Randomized, Subject-Masked, Controlled Clinical Trial Jacklynn F. Sztain, MD, Bahareh Khatibi, MD, Amanda M. Monahan, MD, Engy T. Said, MD, 06/24/2018 on HRKGxsQg0SuoZn4utVXOmk5z97Ja58gACMhy7Bn6J3AP6ghkXyb1tI+UeqR7yrM9qMCtumnaZizf7u+BFX+JxKfAaCZ2GwhaiucBBv+CqffnLy74g548/g== by https://journals.lww.com/anesthesia-analgesia from Downloaded Wendy B. Abramson, MD, Rodney A. Gabriel, MD, MAS, John J. Finneran IV, MD, Richard H. Bellars, MD,* Patrick L. Nguyen, MD,* Scott T. Ball, MD, Francis† B. Gonzales, MD,* Downloaded Sonya S. Ahmed, MD, Michael* C. Donohue, PhD, Jennifer*‡ A. Padwal, BS, and *‡ Brian M. Ilfeld, MD, MS *(Clinical Investigation) * § § from § ∥ ¶ https://journals.lww.com/anesthesia-analgesia BACKGROUND: A continuous adductor canal*‡ block provides analgesia after surgical procedures of the knee. Recent neuroanatomic descriptions of the thigh and knee led us to speculate that local anesthetic deposited in the distal thigh close to the adductor hiatus would provide superior analgesia compared to a more proximal catheter location. We therefore tested the hypothesis that during a continuous adductor canal nerve block, postoperative analgesia would be improved by placing the perineural catheter tip 2–3 cm cephalad to where the femoral artery descends posteri- orly to the adductor hiatus (distal location) compared to a more proximal location at the midpoint between the anterior superior iliac spine and the superior border of the patella (proximal location). by HRKGxsQg0SuoZn4utVXOmk5z97Ja58gACMhy7Bn6J3AP6ghkXyb1tI+UeqR7yrM9qMCtumnaZizf7u+BFX+JxKfAaCZ2GwhaiucBBv+CqffnLy74g548/g== METHODS: Preoperatively, subjects undergoing total knee arthroplasty received an ultrasound- guided perineural catheter inserted either in the proximal or distal location within the adductor canal in a randomized, subject-masked fashion. -

Anomalous Tendinous Contribution to the Adductor Canal by the Adductor Longus Muscle
SHORT COMMUNICATION Anomalous tendinous contribution to the adductor canal by the adductor longus muscle Devon S Boydstun, Jacob F Pfeiffer, Clive C Persaud, Anthony B Olinger Boydstun DS, Pfeiffer JF, Persaud CC, et al. Anomalous tendinous Methods: During routine dissection, one specimen was found to have an contribution to the adductor canal by the adductor longus muscle. Int J Anat abnormal tendinous contribution to the adductor canal. Var. 2017;10(4): 83-84. Results: This tendon arose from the distal portions of adductor longus and ABSTRACT created part of the roof of the canal. Introduction: Classically the adductor canal is made from the fascial Conclusions: The clinical consequences of such an anomaly may include contributions from sartorius, adductor longus, adductor magnus and vastus conditions such as saphenous neuritis, adductor canal compression medialis muscles. The contents of the adductor canal include femoral syndrome, as well as paresthesias along the saphenous nerve distribution. artery, femoral vein, and saphenous nerve. While the femoral artery and vein continue posteriorly through the adductor hiatus, the saphenous nerve Key Words: Adductor Canal; Anomalous tendon; Compressions syndrome; travels all the way through the adductor canal and exits the inferior opening Saphenous neuralgia of the adductor canal. INTRODUCTION he adductor canal is a cone shaped tunnel between the anterior and Tmedial compartments of the thigh through which the femoral artery, femoral vein, and saphenous nerve travel in the distal thigh (1-3). It is bordered anteromedially by the vastoadductor membrane and the Sartorius muscle, anterolaterally by the vastus medialis muscle, and posteriorly by the adductor longus and adductor magnus muscles (3). -

The Nerves of the Adductor Canal and the Innervation of the Knee: An
REGIONAL ANESTHESIA AND ACUTE PAIN Regional Anesthesia & Pain Medicine: first published as 10.1097/AAP.0000000000000389 on 1 May 2016. Downloaded from ORIGINAL ARTICLE The Nerves of the Adductor Canal and the Innervation of the Knee An Anatomic Study David Burckett-St. Laurant, MBBS, FRCA,* Philip Peng, MBBS, FRCPC,†‡ Laura Girón Arango, MD,§ Ahtsham U. Niazi, MBBS, FCARCSI, FRCPC,†‡ Vincent W.S. Chan, MD, FRCPC, FRCA,†‡ Anne Agur, BScOT, MSc, PhD,|| and Anahi Perlas, MD, FRCPC†‡ pain in the first 48 hours after surgery.3 Femoral nerve block, how- Background and Objectives: Adductor canal block contributes to an- ever, may accentuate the quadriceps muscle weakness commonly algesia after total knee arthroplasty. However, controversy exists regarding seen in the postoperative period, as evidenced by its effects on the the target nerves and the ideal site of local anesthetic administration. The Timed-Up-and-Go Test and the 30-Second Chair Stand Test.4,5 aim of this cadaveric study was to identify the trajectory of all nerves that In recent years, an increased interest in expedited care path- course in the adductor canal from their origin to their termination and de- ways and enhanced early mobilization after TKA has driven the scribe their relative contributions to the innervation of the knee joint. search for more peripheral sites of local anesthetic administration Methods: After research ethics board approval, 20 cadaveric lower limbs in an attempt to preserve postoperative quadriceps strength. The were examined using standard dissection technique. Branches of both the adductor canal, also known as the subsartorial or Hunter canal, femoral and obturator nerves were explored along the adductor canal and has been proposed as one such location.6–8 Early data suggest that all branches followed to their termination. -

Anatomy and Clinical Implications of the Ultrasound-Guided Subsartorial Saphenous Nerve Block
Regional Anesthesia & Pain Medicine: first published as 10.1097/AAP.0b013e318220f172 on 1 June 2011. Downloaded from ULTRASOUND ARTICLE Anatomy and Clinical Implications of the Ultrasound-Guided Subsartorial Saphenous Nerve Block Theodosios Saranteas, MD, PhD,* George Anagnostis, MD,* Tilemachos Paraskeuopoulos, PhD,Þ Dimitrios Koulalis, MD,þ Zinon Kokkalis, MD,þ Mariza Nakou, MD,* Sofia Anagnostopoulou, PhD,Þ and Georgia Kostopanagiotou, PhD* landmark, whereas Krombach and Gray7 described a more dis- Background: We evaluated the anatomic basis and the clinical results tal approach, 5 to 7 cm proximal to the popliteal crease and of an ultrasound-guided saphenous nerve block close to the level of the performed a trans-sartorial approach to saphenous nerve block. nerve’s exit from the inferior foramina of the adductor canal. Manickam et al8 also performed a descriptive study to evaluate Methods: The anatomic study was conducted in 11 knees of formalin- the efficacy of an ultrasound-guided saphenous nerve block tech- preserved cadavers in which the saphenous nerve was dissected from nique at the distal part of adductor canal. The authors identified the near its exit from the inferior foramina of the adductor canal. The clinical saphenous nerve within the adductor canal and found that with study was conducted in 23 volunteers. Using a linear probe, the femoral this technique the nerve was blocked successfully in all 20 of vessels and the sartorius muscle were depicted in short-axis view at the their patients. level where the saphenous nerve exits the inferior foramina of the ad- The adductor canal is an aponeurotic tunnel in the middle ductor canal.