Would Predatory Drillhole Frequency on Chione Spp. Increase Under the Suggested Climate Change Scenario? Comparing Pleistocene and Modern Rhodolith Beds
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Chec List Bivalves of the São Sebastião Channel, North Coast Of
Check List 10(1): 97–105, 2014 © 2014 Check List and Authors Chec List ISSN 1809-127X (available at www.checklist.org.br) Journal of species lists and distribution Bivalves of the São Sebastião Channel, north coast of the PECIES S São Paulo State, Brazil OF Lenita de Freitas Tallarico 1*, Flávio Dias Passos 2, Fabrizio Marcondes Machado 3, Ariane Campos 1, ISTS 1 1,4 L Shirlei Maria Recco-Pimentel and Gisele Orlandi Introíni 1 Universidade Estadual de Campinas, Instituto de Biologia, Departamento de Biologia Estrutural e Funcional. R. Charles Darwin, s/n - Bloco N, Caixa Postal 6109. CEP 13083-863. Campinas, SP, Brazil. 2 Universidade Estadual de Campinas, Instituto de Biologia, Departamento de Biologia Animal. Rua Monteiro Lobato, 255, Caixa Postal 6109. CEP 13083-970. Campinas, SP, Brazil. 3 Programas de Pós-Graduação em Ecologia e Biologia Animal, Instituto de Biologia, Universidade Estadual de Campinas. R. Bertrand Russell, s/n, Caixa Postal 6109, CEP 13083-970. Campinas, SP, Brazil. 4 Universidade Federal de Ciências da Saúde de Porto Alegre, Departamento de Ciências Básicas da Saúde. R. Sarmento Leite, 245. CEP 90050-170. Porto Alegre, RS, Brazil. * Corresponding author. E-mail: [email protected] Abstract: The north coast of the São Paulo State, Brazil, presents great bivalve diversity, but knowledge about these organisms, especially species living subtidally, remains scarce. Based on collections made between 2010 and 2012, the present work provides a species list of bivalves inhabiting the intertidal and subtidal zones of the São Sebastião Channel. Altogether, 388 living specimens were collected, belonging to 52 species of 34 genera, grouped in 18 families. -

Induccion Al Desove Y Desarrollo Larval Del Molusco Bivalvo Chione Cancellata
Induccion al Desove y Desarrollo Larval del Molusco Bivalvo Chione cancellata JOSE RENGEL1, LUGO GUELMELIT2, LUIS TORRES2, y CARL HOLUIS MARIN 1Universidad Nacional Experimental Francisco De Complejo Docente El Sabino, Prolongacion Tachira, Sector Universita- rio Punto Fijo, Falcon 4102 Venezuela. 2Universidad Nacional Experimental Francisco De Miranda, Programa De Ing. Pesquera Complejo Docente El Sabino, Prolongacion Tachira, Sector Universidad, Punto Fijo, Falcon 4102 Venezuela RESUMEN El guacuco, Chione cancellata, es una de las especies de moluscos bivalvos de mayor importancia comercial en la costas de la Bahía de Amuay. La mayoría de los pobladores de la zona, viven de su extracción y comercialización. Hasta el momento no se tienen información sobre el desarrollo larval de esta especie, para ser explotado en un futuro cultivo. Por tal motivo, se realizó la inducción al desove de este molusco, utilizando como técnica el choque térmico y descripción de su desarrollo larval. Se obtuvo con éxito el desove después de tres horas de tratamiento térmico y los embriones obtenidos, fueron colocadas y mantenidas en recipientes de 18 L con 15 L de agua de mar filtrada y esterilizadas a 35 UPS y temperatura promedio de 27 ºC, con recambio del 100 % del agua, cada 24 horas. Las larvas se alimentaron con la microalgas Chaetoceros calcitrans, Nannocloropsis sp., y Tretaselmis sp a una concentración de 20.000 cel./ml, de cada una. El desarrollo embrionario del Chione sp. se generó con toda normalidad, alcanzando todas sus fases larvales de la siguiente -

2016 Tese Vprocha.Pdf
0 UNIVERSIDADE FEDERAL DO CEARÁ – UFC INSTITUTO DE CIÊNCIAS DO MAR – LABOMAR PROGRAMA DE PÓS-GRADUAÇÃO EM CIÊNCIAS MARINHAS TROPICAIS VALESCA PAULA ROCHA FILOGENIA MOROLÓGICA E MOLECULAR E ASPECTOS BIOGEOGRÁFICOS DA SUBFAMÍLIA CHIONINAE (BIVALVIA:VENERIDAE) FORTALEZA 2016 1 Dados Internacionais de Catalogação na Publicação Universidade Federal do Ceará Biblioteca Rui Simões de Menezes R577f Rocha, Valesca Paula. Filogenia morfológica e molecular e aspectos biogeográficos da subfamília chioninae (Bivalvia:veneridae). – 2016. 121f.: il. color., enc. ; 30 cm. Tese (doutorado) – Universidade Federal do Ceará, Instituto de Ciências do Mar, Programa de Pós-Graduação em Ciências Marinhas Tropicais, Fortaleza, 2016. Área de Concentração: Utilização e Manejo de Ecossistemas Marinhos e Estuarinos. Orientação: Profª. Drª. Helena Matthews Cascon. Coorientadora: Profª. Drª. Cristiane Xerez Barroso. 1. Conchas - Anatomia. 2. Molusco - Evolução. 3. Bivalvia. 4. Biogeográficos. I. Título. CDD 594.11 2 VALESCA PAULA ROCHA Filogenia Morfológica e Molecular e Aspectos Biogeográficos da Subfamília Chioninae (Bivalvia:Veneridae) Tese submetida à Coordenação do curso de Pós- Graduação em Ciências Marinhas Tropicais do LABOMAR/UFC, como requisito parcial para a obtenção do grau de Doutor em Ciências Marinhas Tropicais. Orientadora: Prof.ª. Drª. Helena Matthews Cascon. Coorientadora: Drª. Cristiane Xerez Barroso FORTALEZA 2016 3 Valesca Paula Rocha Filogenia Morfológica e Molecular e Aspectos Biogeográficos da Subfamília Chioninae (Bivalvia:Veneridae) Tese submetida à Coordenação do curso de Pós-Graduação em Ciências Marinhas Tropicais do LABOMAR /UFC, como requisito parcial para a obtenção do grau de Doutor em Ciências Marinhas Tropicais. Aprovada em 20 de maio de 2016 BANCA EXAMINADORA Coorientadora 4 À minha vó Neusa (in memoriam), que me ensinou seguir firme.. -

Molluscs (Mollusca: Gastropoda, Bivalvia, Polyplacophora)
Gulf of Mexico Science Volume 34 Article 4 Number 1 Number 1/2 (Combined Issue) 2018 Molluscs (Mollusca: Gastropoda, Bivalvia, Polyplacophora) of Laguna Madre, Tamaulipas, Mexico: Spatial and Temporal Distribution Martha Reguero Universidad Nacional Autónoma de México Andrea Raz-Guzmán Universidad Nacional Autónoma de México DOI: 10.18785/goms.3401.04 Follow this and additional works at: https://aquila.usm.edu/goms Recommended Citation Reguero, M. and A. Raz-Guzmán. 2018. Molluscs (Mollusca: Gastropoda, Bivalvia, Polyplacophora) of Laguna Madre, Tamaulipas, Mexico: Spatial and Temporal Distribution. Gulf of Mexico Science 34 (1). Retrieved from https://aquila.usm.edu/goms/vol34/iss1/4 This Article is brought to you for free and open access by The Aquila Digital Community. It has been accepted for inclusion in Gulf of Mexico Science by an authorized editor of The Aquila Digital Community. For more information, please contact [email protected]. Reguero and Raz-Guzmán: Molluscs (Mollusca: Gastropoda, Bivalvia, Polyplacophora) of Lagu Gulf of Mexico Science, 2018(1), pp. 32–55 Molluscs (Mollusca: Gastropoda, Bivalvia, Polyplacophora) of Laguna Madre, Tamaulipas, Mexico: Spatial and Temporal Distribution MARTHA REGUERO AND ANDREA RAZ-GUZMA´ N Molluscs were collected in Laguna Madre from seagrass beds, macroalgae, and bare substrates with a Renfro beam net and an otter trawl. The species list includes 96 species and 48 families. Six species are dominant (Bittiolum varium, Costoanachis semiplicata, Brachidontes exustus, Crassostrea virginica, Chione cancellata, and Mulinia lateralis) and 25 are commercially important (e.g., Strombus alatus, Busycoarctum coarctatum, Triplofusus giganteus, Anadara transversa, Noetia ponderosa, Brachidontes exustus, Crassostrea virginica, Argopecten irradians, Argopecten gibbus, Chione cancellata, Mercenaria campechiensis, and Rangia flexuosa). -

Embryonic and Larval Development of Ensis Arcuatus (Jeffreys, 1865) (Bivalvia: Pharidae)
EMBRYONIC AND LARVAL DEVELOPMENT OF ENSIS ARCUATUS (JEFFREYS, 1865) (BIVALVIA: PHARIDAE) FIZ DA COSTA, SUSANA DARRIBA AND DOROTEA MARTI´NEZ-PATIN˜O Centro de Investigacio´ns Marin˜as, Consellerı´a de Pesca e Asuntos Marı´timos, Xunta de Galicia, Apdo. 94, 27700 Ribadeo, Lugo, Spain (Received 5 December 2006; accepted 19 November 2007) ABSTRACT The razor clam Ensis arcuatus (Jeffreys, 1865) is distributed from Norway to Spain and along the British coast, where it lives buried in sand in low intertidal and subtidal areas. This work is the first study to research the embryology and larval development of this species of razor clam, using light and scanning electron microscopy. A new method, consisting of changing water levels using tide simulations with brief Downloaded from https://academic.oup.com/mollus/article/74/2/103/1161011 by guest on 23 September 2021 dry periods, was developed to induce spawning in this species. The blastula was the first motile stage and in the gastrula stage the vitelline coat was lost. The shell field appeared in the late gastrula. The trocho- phore developed by about 19 h post-fertilization (hpf) (198C). At 30 hpf the D-shaped larva showed a developed digestive system consisting of a mouth, a foregut, a digestive gland followed by an intestine and an anus. Larvae spontaneously settled after 20 days at a length of 378 mm. INTRODUCTION following families: Mytilidae (Redfearn, Chanley & Chanley, 1986; Fuller & Lutz, 1989; Bellolio, Toledo & Dupre´, 1996; Ensis arcuatus (Jeffreys, 1865) is the most abundant species of Hanyu et al., 2001), Ostreidae (Le Pennec & Coatanea, 1985; Pharidae in Spain. -

THE FESTIVUS ISSN: 0738-9388 a Publication of the San Diego Shell Club
(?mo< . fn>% Vo I. 12 ' 2 ? ''f/ . ) QUfrl THE FESTIVUS ISSN: 0738-9388 A publication of the San Diego Shell Club Volume: XXII January 11, 1990 Number: 1 CLUB OFFICERS SCIENTIFIC REVIEW BOARD President Kim Hutsell R. Tucker Abbott Vice President David K. Mulliner American Malacologists Secretary (Corres. ) Richard Negus Eugene V. Coan Secretary (Record. Wayne Reed Research Associate Treasurer Margaret Mulliner California Academy of Sciences Anthony D’Attilio FESTIVUS STAFF 2415 29th Street Editor Carole M. Hertz San Diego California 92104 Photographer David K. Mulliner } Douglas J. Eernisse MEMBERSHIP AND SUBSCRIPTION University of Michigan Annual dues are payable to San Diego William K. Emerson Shell Club. Single member: $10.00; American Museum of Natural History Family membership: $12.00; Terrence M. Gosliner Overseas (surface mail): $12.00; California Academy of Sciences Overseas (air mail): $25.00. James H. McLean Address all correspondence to the Los Angeles County Museum San Diego Shell Club, Inc., c/o 3883 of Natural History Mt. Blackburn Ave., San Diego, CA 92111 Barry Roth Research Associate Single copies of this issue: $5.00. Santa Barbara Museum of Natural History Postage is additional. Emily H. Vokes Tulane University The Festivus is published monthly except December. The publication Meeting date: third Thursday, 7:30 PM, date appears on the masthead above. Room 104, Casa Del Prado, Balboa Park. PROGRAM TRAVELING THE EAST COAST OF AUSTRALIA Jules and Carole Hertz will present a slide program on their recent three week trip to Queensland and Sydney. They will also bring a display of shells they collected Slides of the Club Christmas party will also be shown. -

Embryonic Development of the Tropical Bivalve Tivela Mactroides (Born, 1778) (Veneridae: Subfamily Meretricinae): a SEM Study
Cah. Biol. Mar. (2006) 47 : 243-251 Embryonic development of the tropical bivalve Tivela mactroides (Born, 1778) (Veneridae: subfamily Meretricinae): a SEM study Thomas SILBERFELD and Olivier GROS* UMR 7138 Systématique-Adaptation-Evolution, équipe Symbiose Université des Antilles et de la Guyane, U.F.R des Sciences Exactes et Naturelles. Département de Biologie. 97159 Pointe-à-Pitre Cedex, Guadeloupe (France). *Corresponding author: Tel 590 48 92 13, Fax: 590 48 92 19, E-mail [email protected] Abstract: The embryonic development of Tivela mactroides, from fertilization to straight-hinge veliger D-stage larva occurs in 18 hours at 25°C. Scanning electronic observations show that morphogenetic processes result in a gastrula with two depressions 4 hours after fertilization (T0 + 4h). Two hours later, one depression, located at the animal pole, develops into an open cave, the floor of which becomes the shell field located below the lower face of the prototrochal pad. The invagination located at the vegetal pole features the blastopore. At T0 + 6h, the late gastrula has differentiated into a typi- cal motile trochophore with a shell field synthetizing the organic part of the shell. At T0 + 8h, the shell field, located between the prototroch and the telotroch, appears as a saddle-shaped region with a wrinkled surface extending on both sides of the embryo, establishing bilateral symmetry. At T0 + 12h, the prototroch slides toward the anterior region by outgrowth of the shell material. At T0 + 18h, the prodissoconch I formation is completed and the D-stage larvae possess a calcified shell. At this stage of development, the functional velum is composed of four bands of cilia. -

The Evolution of Extreme Longevity in Modern and Fossil Bivalves
Syracuse University SURFACE Dissertations - ALL SURFACE August 2016 The evolution of extreme longevity in modern and fossil bivalves David Kelton Moss Syracuse University Follow this and additional works at: https://surface.syr.edu/etd Part of the Physical Sciences and Mathematics Commons Recommended Citation Moss, David Kelton, "The evolution of extreme longevity in modern and fossil bivalves" (2016). Dissertations - ALL. 662. https://surface.syr.edu/etd/662 This Dissertation is brought to you for free and open access by the SURFACE at SURFACE. It has been accepted for inclusion in Dissertations - ALL by an authorized administrator of SURFACE. For more information, please contact [email protected]. Abstract: The factors involved in promoting long life are extremely intriguing from a human perspective. In part by confronting our own mortality, we have a desire to understand why some organisms live for centuries and others only a matter of days or weeks. What are the factors involved in promoting long life? Not only are questions of lifespan significant from a human perspective, but they are also important from a paleontological one. Most studies of evolution in the fossil record examine changes in the size and the shape of organisms through time. Size and shape are in part a function of life history parameters like lifespan and growth rate, but so far little work has been done on either in the fossil record. The shells of bivavled mollusks may provide an avenue to do just that. Bivalves, much like trees, record their size at each year of life in their shells. In other words, bivalve shells record not only lifespan, but also growth rate. -
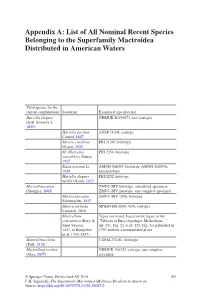
List of All Nominal Recent Species Belonging to the Superfamily Mactroidea Distributed in American Waters
Appendix A: List of All Nominal Recent Species Belonging to the Superfamily Mactroidea Distributed in American Waters Valid species (in the current combination) Synonym Examined type material Harvella elegans NHMUK 20190673, two syntypes (G.B. Sowerby I, 1825) Harvella pacifica ANSP 51308, syntype Conrad, 1867 Mactra estrellana PRI 21265, holotype Olsson, 1922 M. (Harvella) PRI 2354, holotype sanctiblasii Maury, 1925 Raeta maxima Li, AMNH 268093, lectotype; AMNH 268093a, 1930 paralectotype Harvella elegans PRI 2252, holotype tucilla Olsson, 1932 Mactrellona alata ZMUC-BIV, holotype, articulated specimen; (Spengler, 1802) ZMUC-BIV, paratype, one complete specimen Mactra laevigata ZMUC-BIV 1036, holotype Schumacher, 1817 Mactra carinata MNHN-IM-2000-7038, syntypes Lamarck, 1818 Mactrellona Types not found, based on the figure of the concentrica (Bory de “Tableau of Encyclopedique Methodique…” Saint Vincent, (pl. 251, Fig. 2a, b, pl. 252, Fig. 2c) published in 1827, in Bruguière 1797 without a nomenclatorial act et al. 1791–1827) Mactrellona clisia USNM 271481, holotype (Dall, 1915) Mactrellona exoleta NHMUK 196327, syntype, one complete (Gray, 1837) specimen © Springer Nature Switzerland AG 2019 103 J. H. Signorelli, The Superfamily Mactroidea (Mollusca:Bivalvia) in American Waters, https://doi.org/10.1007/978-3-030-29097-9 104 Appendix A: List of All Nominal Recent Species Belonging to the Superfamily… Valid species (in the current combination) Synonym Examined type material Lutraria ventricosa MCZ 169451, holotype; MCZ 169452, paratype; -

Inventory of Mollusks from the Estuary of the Paraíba River in Northeastern Brazil
Biota Neotropica 17(1): e20160239, 2017 www.scielo.br/bn ISSN 1676-0611 (online edition) inventory Inventory of mollusks from the estuary of the Paraíba River in northeastern Brazil Silvio Felipe Barbosa Lima1*, Rudá Amorim Lucena2, Galdênia Menezes Santos3, José Weverton Souza3, Martin Lindsey Christoffersen2, Carmen Regina Guimarães4 & Geraldo Semer Oliveira4 1Universidade Federal de Campina Grande, Unidade Acadêmica de Ciências Exatas e da Natureza, Centro de Formação de Professores, Cajazeiras, PB, Brazil 2Universidade Federal da Paraíba, Departamento de Sistemática e Ecologia, João Pessoa, PB, Brazil 3Universidade Federal de Sergipe, Departamento de Ecologia, São Cristóvão, SE, Brazil 4Universidade Federal de Sergipe, Departamento de Biologia, São Cristóvão, SE, Brazil *Corresponding author: Silvio Felipe Lima, e-mail: [email protected] LIMA, S.F.B., LUCENA, R.A., SANTOS, G.M., SOUZA, J.W., CHRISTOFFERSEN, M.L., GUIMARÃES, C.R., OLIVEIRA, G.S. Inventory of mollusks from the estuary of the Paraíba River in northeastern Brazil. Biota Neotropica. 17(1): e20160239. http://dx.doi.org/10.1590/1676-0611-BN-2016-0239 Abstract: Coastal ecosystems of northeastern Brazil have important biodiversity with regard to marine mollusks, which are insufficiently studied. Here we provide an inventory of mollusks from two sites in the estuary of the Paraíba River. Mollusks were collected in 2014 and 2016 on the coast and sandbanks located on the properties of Treze de Maio and Costinha de Santo Antônio. The malacofaunal survey identified 12 families, 20 genera and 21 species of bivalves, 17 families, 19 genera and 20 species of gastropods and one species of cephalopod. Bivalves of the family Veneridae Rafinesque, 1815 were the most representative, with a total of five species. -

Ciclo Reproductivo De La ALMEJA ROÑOSA, Chione Californiensis
Ciencias Marinas (1993), 19(1):15-28. http://dx.doi.org/10.7773/cm.v19i1.923 CICLO REPRODUCTIVO DE LA ALMEJA ROÑOSA, Chione cdifomiensis (BRODERIP, 1835), EN BAHIA MAGDALENA, BAJA CALIFORNIA SUR, MEXICO REPRODUCTIVE CYCLE OF THE CLAM Chione cdifomiensis (BRODEIUP, 1835) IN BAHIA MAGDALENA, BAJA CALIFORNIA SUR, MEXICO Federico García-Domínguez* Gustavo García-Melgar Pedro González-Ramírez* Centro Interdisciplinario de Ciencias Marinas Instituto Politécnico Nacional Apartado Postal 592 La Paz, B.C.S., 23000 México Recibido en enero de 1992; aceptado en mayo de 1992 RESUMEN Se recolectaronmensualmente, entre mayo de 1988 y septiembre de 1989, ejemplares adultos de la almeja roñosa, Chione califomiensis (Broderip, 1835), en una población de la zona entre mareas situada en Puerto San Carlos, Bahía Magdalena, B.C.S. El desarrollo gonádico fue analizado utilizando las técnicas histológicas usuales. Las fases del desarrollo fueron categorizadas en cinco estadios: indiferenciación, gametogénesis, madurez, desove y postdesove. Tanto las hembras como los machos presentaron las mismas fases. El desove de la población fue continuo durante cuatro meses en 1988 (junio a septiembre) y al menos durante seis meses en 1989 (abril a septiembre). Ambos años con un máximo de actividad reproductora en agosto. ABSTRACT Adult clams Chione califomiensis (Broderip, 1835) were sampled monthly between May 1988 and September 1989, from intertidal populations in Puerto San Carlos, Bahía Magdalena, Baja California Sur. Gonadal development was monitored using standard histological methods. Observed gametogenic progression was categorized by five stages: inactive, gametogenesis, ripe, spawning and spent. Both male and female clams displayed the same stages. Spawning in the population occurred continuously for four months in 1988 (June to September) and for six months in 1989 (April to September), both years with a peak of spawning activity in August. -

Mollusk Community of the Punta De Piedras Coastal Lagoon System, Isla De Margarita, Venezuela
Page 512 62nd Gulf and Caribbean Fisheries Institute Mollusk Community of the Punta de Piedras Coastal Lagoon System, Isla de Margarita, Venezuela FRANCIS MASS1 and JUAN C. CAPELO2 1Wildlife Conservation Society, Fundación la Salle de Ciencias Naturales, Estación de Investigaciones Hidrobiológicas de Guayana, San Félix, Bolívar, Venezuela. 2Estación de Investigaciones Marinas de Margarita, Fundación La Salle de Ciencias Naturales, Isla de Margarita, Punta de Piedras, Nueva Esparta, Venezuela ABSTRACT In this work we argue the richness, abundance, and distribution of the mollusks community from the Punta de Piedras coastal Lagoon system, Southern Isla de Margarita, Nueva Esparta state, Venezuela. The specimens were collected using dredges, PVC nucleuses, sieves, spatulas, tweezers, plastic bags, and by hand. Sampled habitats were realized mainly on streams and secondary canals and mangrove roots, submerged trunks, soft funds, artificial rocky coasts and Thalassia testudinum meadows. We collected 2467 specimens, distributed in 59 families, 87 genera and 131 species, representatives of the classes Bivalvia, Gastropoda, Polyplacophora and Cephalopoda. The gastropods were the dominant class with 71 species, being Bulla striata the most common species, found in all localities visited. The most abundant species were Persicula interruptolineata, Littorina lineolata, Crepidula plana, Chione cancellata and Crassostrea rhizophorae. The most richness habitat was the rocky shores (58 species) and sandy bottom (57 species). We report as new records for Nueva Esparta state the gastropods Cerithiopsis greeni, Agathotoma badia, and Turbonilla pyrrha. We identified four trophy groups (carnivorous, herbivorous, suspensivores and organic particles consumers), of which the suspensivores and carnivores accounted 70% of all species. The most important species used as resource subsistence by local people were Crassostrea rhizophorae, Perna viridis, P.