Three Dimensional Renderings of the Glossopodia of North American Isoetes Ligules
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

WRA Species Report
Family: Selaginellaceae Taxon: Selaginella braunii Synonym: Lycopodioides braunii (Baker) Kuntze Common Name: arborvitae fern Selaginella braunii fo. hieronymi Alderw. Braun's spike-moss Selaginella hieronymi Alderw. Chinese lace-fern spike-moss Selaginella vogelii Mett. treelet spike-moss Questionaire : current 20090513 Assessor: Assessor Designation: H(HPWRA) Status: Assessor Approved Data Entry Person: Assessor WRA Score 7 101 Is the species highly domesticated? y=-3, n=0 n 102 Has the species become naturalized where grown? y=1, n=-1 103 Does the species have weedy races? y=1, n=-1 201 Species suited to tropical or subtropical climate(s) - If island is primarily wet habitat, then (0-low; 1-intermediate; 2- Low substitute "wet tropical" for "tropical or subtropical" high) (See Appendix 2) 202 Quality of climate match data (0-low; 1-intermediate; 2- High high) (See Appendix 2) 203 Broad climate suitability (environmental versatility) y=1, n=0 y 204 Native or naturalized in regions with tropical or subtropical climates y=1, n=0 n 205 Does the species have a history of repeated introductions outside its natural range? y=-2, ?=-1, n=0 y 301 Naturalized beyond native range y = 1*multiplier (see y Appendix 2), n= question 205 302 Garden/amenity/disturbance weed n=0, y = 1*multiplier (see n Appendix 2) 303 Agricultural/forestry/horticultural weed n=0, y = 2*multiplier (see n Appendix 2) 304 Environmental weed n=0, y = 2*multiplier (see n Appendix 2) 305 Congeneric weed n=0, y = 1*multiplier (see y Appendix 2) 401 Produces spines, thorns or -

Selaginellaceae: Traditional Use, Phytochemistry and Pharmacology
MS Editions BOLETIN LATINOAMERICANO Y DEL CARIBE DE PLANTAS MEDICINALES Y AROMÁTICAS 19 (3): 247 - 288 (2020) © / ISSN 0717 7917 / www.blacpma.ms-editions.cl Revisión | Review Selaginellaceae: traditional use, phytochemistry and pharmacology [Selaginellaceae: uso tradicional, fitoquímica y farmacología] Fernanda Priscila Santos Reginaldo, Isabelly Cristina de Matos Costa & Raquel Brandt Giordani College of Pharmacy, Pharmacy Department. University of Rio Grande do Norte, Natal, RN, Brazil. Contactos | Contacts: Raquel Brandt GIORDANI - E-mail address: [email protected] Abstract: Selaginella is the only genus from Selaginellaceae, and it is considered a key factor in studying evolution. The family managed to survive the many biotic and abiotic pressures during the last 400 million years. The purpose of this review is to provide an up-to-date overview of Selaginella in order to recognize their potential and evaluate future research opportunities. Carbohydrates, pigments, steroids, phenolic derivatives, mainly flavonoids, and alkaloids are the main natural products in Selaginella. A wide spectrum of in vitro and in vivo pharmacological activities, some of them pointed out by folk medicine, has been reported. Future studies should afford valuable new data on better explore the biological potential of the flavonoid amentoflavone and their derivatives as chemical bioactive entities; develop studies about toxicity and, finally, concentrate efforts on elucidate mechanisms of action for biological properties already reported. Keywords: Selaginella; Natural Products; Overview. Resumen: Selaginella es el único género de Selaginellaceae, y se considera un factor clave en el estudio de la evolución. La familia logró sobrevivir a las muchas presiones bióticas y abióticas durante los últimos 400 millones de años. -

Application of DNA Flow Cytometry to Aid Species Delimitation in Isoetes Author(S): Jay F
Application of DNA Flow Cytometry to Aid Species Delimitation in Isoetes Author(s): Jay F. Bolin, Carmony L. Hartwig, Peter Schafran, and Slavko Komarnytsky Source: Castanea, 83(1):38-47. Published By: Southern Appalachian Botanical Society https://doi.org/10.2179/16-120 URL: http://www.bioone.org/doi/full/10.2179/16-120 BioOne (www.bioone.org) is a nonprofit, online aggregation of core research in the biological, ecological, and environmental sciences. BioOne provides a sustainable online platform for over 170 journals and books published by nonprofit societies, associations, museums, institutions, and presses. Your use of this PDF, the BioOne Web site, and all posted and associated content indicates your acceptance of BioOne’s Terms of Use, available at www.bioone.org/page/ terms_of_use. Usage of BioOne content is strictly limited to personal, educational, and non-commercial use. Commercial inquiries or rights and permissions requests should be directed to the individual publisher as copyright holder. BioOne sees sustainable scholarly publishing as an inherently collaborative enterprise connecting authors, nonprofit publishers, academic institutions, research libraries, and research funders in the common goal of maximizing access to critical research. CASTANEA 83(1): 38–47. DECEMBER Copyright 2018 Southern Appalachian Botanical Society Application of DNA Flow Cytometry to Aid Species Delimitation in Isoetes Jay F. Bolin,1*CarmonyL.Hartwig,1 Peter Schafran,2 andSlavkoKomarnytsky3,4 1Department of Biology, Catawba College, Salisbury, North Carolina 28144 2Department of Biological Sciences, Old Dominion University, Norfolk, Virginia 23529 3Plants for Human Health Institute, North Carolina State University, Kannapolis, North Carolina 28081 4Department of Food, Bioprocessing, and Nutrition Sciences, North Carolina State University, Raleigh, North Carolina 27695 ABSTRACT The genus Isoetes is known for morphological convergence and a relative paucity of useful gross morphological characters for identification. -

Bibliography of Isoetes
BIBLIOGRAPHY OF ISOETES ALLEN, B.M. 1975. A note on the distribution of Isoetes in the Cadiz Province, Spain. Fern Gaz. (U.K.) 11 (2-3): 163-164 (1975). ALONSO, PAZ, E. 1989. Notas sobre plantas nuevas o interesantes para la flora Uruguaya: 1. (Notes on new or interesting plants for the Uruguayan flora: 1.) Comun. Bot. Mus. Hist. Nat. Montevideo 5 (91): 1-4 (1989) - Isoetes pp.2-3 ALSTON, A.H.G. 1982. Isoetaceae: 1. In Steenis, C.G.G.J. van, Holttum, R. E., eds. Flora Malesiana, series 2. Pteridophytes, volume 1. The Hague, Martinus Nijhoff, Dr. W. Junk Publ. 62-64 (1982)- illus., chrom. nos., key. ANDREIS, C., RODONDI, G. 1987. Alcune stazioni di Isoetes echinospora Dur. nel Bresciano e osservazioni al SEM delle spore delle Isoetes della flora Italica. Natura Bresciana no.23: 119-130 (1986 publ. 1987) - illus., maps. 4, ANTHONY, N.C., & E.A. SCHELPE, 1985. Two new taxa and a new combination in southern African Pteridophyta. Bothalia, 15 (3 & 4): 554-555 (1985) ARREGUIN-SANCHEZ, M., 1986. Nuevos registros y taxa interesantes de pteridofitas del Valle de Mexico. (Isoetaceae, Psilotaceae y Selaginellaceae) Phytologia 59 (7): 451-453 (1986) ASH, S., & K.B. PIGG. 1991. A new Jurassic Isoetites (Isoetales) from the Wallowa Terrane in Hells Canyon Oregon and Idaho. Amer. J. Bot. 78: 1636-1642. BAJPAI, U., & H.K. MAHESHWARI,1985. EM studies on the megaspores of Isoetes coromandelina. Phytomorphology, 34 (1-4): 226-231 (1984 publ. 1985) - illus. BALDWIN, W.K.W. 1933. The organization of the young sporophyte of Isoetes engelmanni, A. -

Chapter 23: the Early Tracheophytes
Chapter 23 The Early Tracheophytes THE LYCOPHYTES Lycopodium Has a Homosporous Life Cycle Selaginella Has a Heterosporous Life Cycle Heterospory Allows for Greater Parental Investment Isoetes May Be the Only Living Member of the Lepidodendrid Group THE MONILOPHYTES Whisk Ferns Ophioglossalean Ferns Horsetails Marattialean Ferns True Ferns True Fern Sporophytes Typically Have Underground Stems Sexual Reproduction Usually Is Homosporous Fern Have a Variety of Alternative Means of Reproduction Ferns Have Ecological and Economic Importance SUMMARY PLANTS, PEOPLE, AND THE ENVIRONMENT: Sporophyte Prominence and Survival on Land PLANTS, PEOPLE, AND THE ENVIRONMENT: Coal, Smog, and Forest Decline THE OCCUPATION OF THE LAND PLANTS, PEOPLE, AND THE The First Tracheophytes Were ENVIRONMENT: Diversity Among the Ferns Rhyniophytes Tracheophytes Became Increasingly Better PLANTS, PEOPLE, AND THE Adapted to the Terrestrial Environment ENVIRONMENT: Fern Spores Relationships among Early Tracheophytes 1 KEY CONCEPTS 1. Tracheophytes, also called vascular plants, possess lignified water-conducting tissue (xylem). Approximately 14,000 species of tracheophytes reproduce by releasing spores and do not make seeds. These are sometimes called seedless vascular plants. Tracheophytes differ from bryophytes in possessing branched sporophytes that are dominant in the life cycle. These sporophytes are more tolerant of life on dry land than those of bryophytes because water movement is controlled by strongly lignified vascular tissue, stomata, and an extensive cuticle. The gametophytes, however still require a seasonally wet habitat, and water outside the plant is essential for the movement of sperm from antheridia to archegonia. 2. The rhyniophytes were the first tracheophytes. They consisted of dichotomously branching axes, lacking roots and leaves. They are all extinct. -
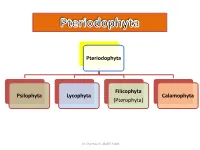
Pteriodophyta Psilophyta Lycophyta Filicophyta (Pterophyta) Calamophyta
Pteriodophyta Filicophyta Psilophyta Lycophyta Calamophyta (Pterophyta) Dr. Shaimaa N. Abd El-Fatah Class(2): Lycophyta Subclass(1): Homospora (Eligulatae) Order: Lycopodiales Family: Lycopodiaceae e.g. Lycopodium Subclass(2): Heterospora (Ligulatae) Order: Selaginellales Family: Selaginellaceae e.g. Selaginella Dr. Shaimaa N. Abd El-Fatah Phylum: Lepidophyta • It is characterized by: 1. The plant is differentiated into stem, leaves and roots. 2. Leaves are microphyllous (small, one vein and with no leaf gap). 3. The stele is protostele, siphonostele or polystele. 4. Protoxylem is exarch (ranging from a complete external cylinder to a polyarch stele in which there are many protoxylem ribs). 5. Sporangia are solitary, carried on special leaves (sporophylls). Sporophylls are usually collected in strobili. Dr. Shaimaa N. Abd El-Fatah . Among this phylum there are two independent evolutionary lines Heterospora (Ligulatae) Homospora (Eligulatae) • Characterized by presence • Eligulate (ligule lacking). of ligule (small outgrowth on • Homosporous (one type of the upper surface of the spores). leaf). • Heterosporous character (2 types of spores: microspores-- small, give rise to male gametophyte. & megaspores– larger, give rise to female gametophyte.) Dr. Shaimaa N. Abd El-Fatah • The phylum includes one class: Lycopodinae. • The class is classified into 4 orders: • Plants belong to the first order are homosporae (eligulatae), while the remaining three are heterosporae (ligulatae). Dr. Shaimaa N. Abd El-Fatah Class(2): Lycophyta Subclass(1): Homospora Subclass(2): Heterospora (Eligulatae) (Ligulatae) Order: Lycopodiales Order: Selaginellales Family: Lycopodiaceae Family: Selaginellaceae e.g. Lycopodium e.g. Selaginella Dr. Shaimaa N. Abd El-Fatah Lycopodiales This order is characterized by: 1. Homosporous and eligulatae. 2. Herbaceous without secondary growth. -

Spore Dispersal of Selaginella Denticulata, S. Helvetica, and S
Zurich Open Repository and Archive University of Zurich Main Library Strickhofstrasse 39 CH-8057 Zurich www.zora.uzh.ch Year: 2020 Spore dispersal of Selaginella denticulata, S. helvetica, and S. selaginoides, and the significance of heterospory in Selaginellacae Schneller, Jakob ; Kessler, Michael DOI: https://doi.org/10.1640/0002-8444-110.2.58 Posted at the Zurich Open Repository and Archive, University of Zurich ZORA URL: https://doi.org/10.5167/uzh-187856 Journal Article Published Version Originally published at: Schneller, Jakob; Kessler, Michael (2020). Spore dispersal of Selaginella denticulata, S. helvetica, and S. selaginoides, and the significance of heterospory in Selaginellacae. American Fern Journal, 110(2):58-65. DOI: https://doi.org/10.1640/0002-8444-110.2.58 Spore dispersal of Selaginella denticulata, S. helvetica, and S. selaginoides, and the significance of heterospory in Selaginellacae Authors: Schneller, Jakob, and Kessler, Michael Source: American Fern Journal, 110(2) : 58-65 Published By: The American Fern Society URL: https://doi.org/10.1640/0002-8444-110.2.58 BioOne Complete (complete.BioOne.org) is a full-text database of 200 subscribed and open-access titles in the biological, ecological, and environmental sciences published by nonprofit societies, associations, museums, institutions, and presses. Your use of this PDF, the BioOne Complete website, and all posted and associated content indicates your acceptance of BioOne’s Terms of Use, available at www.bioone.org/terms-of-use. Usage of BioOne Complete content is strictly limited to personal, educational, and non - commercial use. Commercial inquiries or rights and permissions requests should be directed to the individual publisher as copyright holder. -

Phyton Annales Rei Botanicae
©Verlag Ferdinand Berger & Söhne Ges.m.b.H., Horn, Austria, download unter www.biologiezentrum.at PHYTON ANNALES REI BOTANICAE VOL. 45, FASC. 1 PAG. 1--144 30. 6. 2005 Phyton (Horn, Austria) Vol. 45 Fasc. 1 1-8 30. 6. 2005 The Gametophyte-Sporophyte Junction in Selaginella martensii SPRING (Selaginellales, Lycopodiophyta) By Hartmut H. HILGER*), Nancy KAPUSKAR**) and Wolfgang FREY*) With 6 Figures Received August 20, 2004 Key words: Lycopodiophyta, Pteridophyta, Selaginella, Selaginellales. - Anatomy;omy,, gametophyte-sporophytgame e junction, placental space. - Electron microscopy. Summary HILGER H. H., KAPUSKAR N. & FREY W. 2005. The gametophyte-sporophyte junction in Selaginella martensii SPRING (Selaginellales, Lycopodiophyta). - Phyton (Horn, Austria) 45 (1): 1-8, with 6 figures. - English with German summary. The gametophyte-sporophyte junction in Selaginella martensii (Selaginellales, Lycopodiophyta) consists of a sporophytic conical foot embedded in the maternal gametophytic tissue. Both generations are separated by a narrow placental space *) Prof. Dr. H. H. HILGER, Prof. Dr. W. FREY, Institut für Biologie - Systematische Botanik und Pflanzengeographie - der Freien Universität Berlin, Altensteinstrae 6, D-14195 Berlin, Germany; e-mail: [email protected], [email protected] berlin.de **) Dr. N. KAPUSKAR, Institut für Spezielle Botanik und Botanischer Garten der Johannes Gutenberg-Universität, Bentzelweg 2, D-55099 Mainz; e-mail: [email protected] ©Verlag Ferdinand Berger & Söhne Ges.m.b.H., Horn, Austria, download unter www.biologiezentrum.at filled with thin-walled collapsed cells of gametophytic origin. Gametophytic and sporophytic placental cells lack wall ingrowths, respectively transfer cells. Neither interdigitation nor intermingling of placental cells nor nacreous thickenings are de- veloped. The structure of the gametophyte-sporophyte junction in Selaginella mar- tensii resembles that of Isoetes boliviensis, the second lycopodiopside investigated, and thus differs from the junction described for pteridopsides and lycopods. -

Selaginella Wangpeishanii (Selaginellaceae), a New Lycophyte from a Limestone Cave in Guizhou, China
Phytotaxa 164 (3): 195–199 ISSN 1179-3155 (print edition) www.mapress.com/phytotaxa/ Article PHYTOTAXA Copyright © 2014 Magnolia Press ISSN 1179-3163 (online edition) http://dx.doi.org/10.11646/phytotaxa.164.3.5 Selaginella wangpeishanii (Selaginellaceae), a new lycophyte from a limestone cave in Guizhou, China LI-BING ZHANG1 , QING-WEN SUN2 & HAI HE3* 1Chengdu Institute of Biology, Chinese Academy of Sciences, P.O. Box 416, Chengdu, Sichuan 610041, P. R. China and Missouri Botanical Garden, P.O. Box 299, St. Louis, Missouri 63166-0299, U.S.A. 2Department of Pharmacy, Guiyang College of Traditional Chinese Medicine, Guiyang, Guizhou 550002, P. R. China 3College of Life Sciences, Chongqing Normal University, Shapingba, Chongqing 400047, P. R. China *Author for correspondence: e-mail: [email protected] Abstract Selaginella wangpeishanii, a new species of the lycophyte genus Selaginella (Selaginellaceae) from a limestone cave in southern Guizhou, China is described and illustrated. The new species is different from any species in the genus known so far in China by having some ultimate branches bearing microphylls in ascending order of trophophylls, sporophylls or sporophyll-like microphylls, and trophophylls (TST arrangement of microphylls). Morphologically, S. wangpeishanii is most similar to S. gebaueriana, but is distinct by smaller plants and serrulate margins of lateral trophophylls. Selaginella wangpeishanii is currently known only from a single population and is considered to be Critically Endangered (CR), based on IUCN Red List criteria. Key words: Selaginella wangpeishanii, Guizhou, TST arrangement of microphylls, IUCN Red List Introduction Selaginella Beauvois (1804: 478) (Selaginellaceae) is the largest lycophyte genus with about 750 species mainly distributed in tropical and subtropical areas (Jermy 1990). -

Threatened and Endangered Species List
Effective April 15, 2009 - List is subject to revision For a complete list of Tennessee's Rare and Endangered Species, visit the Natural Areas website at http://tennessee.gov/environment/na/ Aquatic and Semi-aquatic Plants and Aquatic Animals with Protected Status State Federal Type Class Order Scientific Name Common Name Status Status Habit Amphibian Amphibia Anura Gyrinophilus gulolineatus Berry Cave Salamander T Amphibian Amphibia Anura Gyrinophilus palleucus Tennessee Cave Salamander T Crustacean Malacostraca Decapoda Cambarus bouchardi Big South Fork Crayfish E Crustacean Malacostraca Decapoda Cambarus cymatilis A Crayfish E Crustacean Malacostraca Decapoda Cambarus deweesae Valley Flame Crayfish E Crustacean Malacostraca Decapoda Cambarus extraneus Chickamauga Crayfish T Crustacean Malacostraca Decapoda Cambarus obeyensis Obey Crayfish T Crustacean Malacostraca Decapoda Cambarus pristinus A Crayfish E Crustacean Malacostraca Decapoda Cambarus williami "Brawley's Fork Crayfish" E Crustacean Malacostraca Decapoda Fallicambarus hortoni Hatchie Burrowing Crayfish E Crustacean Malocostraca Decapoda Orconectes incomptus Tennessee Cave Crayfish E Crustacean Malocostraca Decapoda Orconectes shoupi Nashville Crayfish E LE Crustacean Malocostraca Decapoda Orconectes wrighti A Crayfish E Fern and Fern Ally Filicopsida Polypodiales Dryopteris carthusiana Spinulose Shield Fern T Bogs Fern and Fern Ally Filicopsida Polypodiales Dryopteris cristata Crested Shield-Fern T FACW, OBL, Bogs Fern and Fern Ally Filicopsida Polypodiales Trichomanes boschianum -

Inferring the Evolutionary Reduction of Corm Lobation in Isoëtes Using Bayesian Model- Averaged Ancestral State Reconstruction
UC Berkeley UC Berkeley Previously Published Works Title Inferring the evolutionary reduction of corm lobation in Isoëtes using Bayesian model- averaged ancestral state reconstruction. Permalink https://escholarship.org/uc/item/39h708p5 Journal American journal of botany, 105(2) ISSN 0002-9122 Authors Freund, Forrest D Freyman, William A Rothfels, Carl J Publication Date 2018-02-01 DOI 10.1002/ajb2.1024 Peer reviewed eScholarship.org Powered by the California Digital Library University of California RESEARCH ARTICLE BRIEF COMMUNICATION Inferring the evolutionary reduction of corm lobation in Isoëtes using Bayesian model- averaged ancestral state reconstruction Forrest D. Freund1,2, William A. Freyman1,2, and Carl J. Rothfels1 Manuscript received 26 October 2017; revision accepted 2 January PREMISE OF THE STUDY: Inferring the evolution of characters in Isoëtes has been problematic, 2018. as these plants are morphologically conservative and yet highly variable and homoplasious 1 Department of Integrative Biology, University of California, within that conserved base morphology. However, molecular phylogenies have given us a Berkeley, Berkeley, CA 94720-3140, USA valuable tool for testing hypotheses of character evolution within the genus, such as the 2 Authors for correspondence (e-mail: [email protected], hypothesis of ongoing morphological reductions. [email protected]) Citation: Freund, F. D., W. A. Freyman, and C. J. Rothfels. 2018. METHODS: We examined the reduction in lobe number on the underground trunk, or corm, by Inferring the evolutionary reduction of corm lobation in Isoëtes using combining the most recent molecular phylogeny with morphological descriptions gathered Bayesian model- averaged ancestral state reconstruction. American from the literature and observations of living specimens. -

Common Name: MAT-FORMING QUILLWORT Scientific Name
Common Name: MAT-FORMING QUILLWORT Scientific Name: Isoetes tegetiformans Rury Other Commonly Used Names: Merlin’s-grass Previously Used Scientific Names: none Family: Isoetaceae (quillwort) Rarity Ranks: G1/S1 State Legal Status: Endangered Federal Legal Status: Endangered Federal Wetland Status: OBL Description: Perennial herb forming dense mats in granite outcrop pools. Stem brown, horizontal at or just below the soil surface, often pushing up small hummocks of soil; horizontal stems bear stout, coiled roots near the leaf bases and slender, straight roots near the end of the stem. Leaves usually less than 1½ inches (3 cm) tall, rarely up to 2 ¾ inches (7 cm) tall, very narrow and pointed, in clusters of 4 - 8 leaves along the top of the horizontal stem; leaves hollow with cross partitions. Spores produced in a chamber (sporangium) in the flared leaf base, the chamber completely covered by a translucent membrane (velum). Dozens of tiny, brown female spores may be seen with 30x magnification; minute, dust-sized male spores, which occur on separate leaves, are also present but are indistinguishable without much higher magnification. Similar Species: Quillworts are distinguished from flowering, wetland plants by their spongy leaves with conspicuous cross-walls and by the presence of sporangia in the flared base of the leaves. Piedmont quillwort (Isoetes piedmontana) occurs in muddy seeps and ephemeral pools in erosion pits on granite outcrops. Its leaves are 2¾ - 6 inches (7 - 15 cm) long and have brown to black bases; the sporangia contain larger, distinctly dimpled megaspores that are only partially covered by the velum. Related Rare Species: Black-spored quillwort (Isoetes melanospora) occurs in similar habitats in Georgia and South Carolina but is larger, with larger megaspores, and leaves arranged spirally about the corm (see full species account elsewhere on this website).