Elemental Profiles in Cycas Micronesica Stems
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
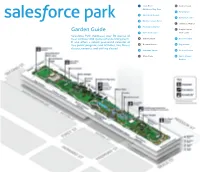
Salesforce Park Garden Guide
Start Here! D Central Lawn Children’s Play Area Garden Guide6 Palm Garden 1 Australian Garden Start Here! D Central Lawn Salesforce Park showcases7 California over Garden 50 species of Children’s Play Area 2 Mediterraneantrees and Basin over 230 species of understory plants. 6 Palm Garden -ã ¼ÜÊ ÊăØÜ ØÊèÜãE úØƀØÊèÃJapanese Maples ¼ÃØ Ê¢ 1 Australian Garden 3 Prehistoric¢ØÕ輫ÕØÊ£ØÂÜÃã«ó«ã«Üŧ¼«¹ĆãÃÜÜ Garden 7 California Garden ¼ÜÜÜŧÊÃØãÜŧÃØ¢ã«Ã£¼ÜÜÜũF Amphitheater Garden Guide 2 Mediterranean Basin 4 Wetland Garden Main Lawn E Japanese Maples Salesforce Park showcases over 50 species of 3 Prehistoric Garden trees and over 230 species of understory plants. A Oak Meadow 8 Desert Garden F Amphitheater It also offers a robust year-round calendar of 4 Wetland Garden Main Lawn free public programs and activities, like fitness B Bamboo Grove 9 Fog Garden Desert Garden classes, concerts, and crafting classes! A Oak Meadow 8 5 Redwood Forest 10 Chilean Garden B Bamboo Grove 9 Fog Garden C Main Plaza 11 South African 10 Chilean Garden Garden 5 Redwood Forest C Main Plaza 11 South African Garden 1 Children’s Australian Play Area Garden ABOUT THE GARDENS The botanist aboard the Endeavor, Sir Joseph Banks, is credited with introducing many plants from Australia to the western world, and many This 5.4 acre park has a layered soil system that plants today bear his name. balances seismic shifting, collects and filters storm- water, and irrigates the gardens. Additionally, the soil Native to eastern Australia, Grass Trees may grow build-up and dense planting help offset the urban only 3 feet in 100 years, and mature plants can be heat island effect by lowering the air temperature. -

Steryl Glucoside Concentration Declines with Cycas Micronesica Seed Age
CSIRO PUBLISHING www.publish.csiro.au/journals/fpb Functional Plant Biology, 2006, 33, 857–862 Steryl glucoside concentration declines with Cycas micronesica seed age Thomas E. MarlerA,C, Vivian LeeB, J. ChungB and Christopher A. ShawB ACNAS-AES, University of Guam, UOG Station, Mangilao, GU 96923, USA. BDepartment of Ophthalmology, University of British Columbia, Vancouver, British Columbia, Canada V6T 1Z4. CCorresponding author. Email: [email protected] Abstract. Neurotoxins contained in the seeds of Cycas micronesica K.D. Hill have been implicated in the Guam neurological disease cluster, amyotrophic lateral sclerosis–parkinsonism dementia complex (ALS–PDC). Some of these neurotoxins remain in the washed cycad seed flour that was historically an important part of the Chamorro diet. Of these, variant steryl glucosides have been identified by us as a possible etiological factor in the disease. In vitro and in vivo animal studies have strongly supported a role for these molecules in some forms of neurodegeneration. As part of a series of studies, we have now determined the concentrations of several steryl glucosides and their sterol precursors as affected by the age of C. micronesica seeds. The concentration of these molecules declined with seed age from 2.0 to 30.5 months. Following log-transformation of both axes, the decline was linear. Similarly, concentration of all but one of the molecules declined with age when samples were restricted to gametophyte tissue. Factors suspected of influencing phenotypic plasticity must be addressed when interpreting plant physiology data. Our results confirm for the first time that tissue age must be documented and reported in cycad seed biochemistry studies to remove ambiguities from results. -

Cycad Aulacaspis Scale
Invasive Insects: Risks and Pathways Project CYCAD AULACASPIS SCALE UPDATED: APRIL 2020 Invasive insects are a huge biosecurity challenge. We profile some of the most harmful insect invaders overseas to show why we must keep them out of Australia. Species Cycad aulacaspis scale / Aulacaspis yasumatsui. Also known as Asian cycad scale. Main impacts Decimates wild cycad populations, kills cultivated cycads. Native range Thailand. Invasive range China, Taiwan, Singapore, Indonesia, Guam, United States, Caribbean Islands, Mexico, France, Ivory Coast1,2. Detected in New Zealand in 2004, but eradicated.2 Main pathways of global spread As a contaminant of traded nursery material (cycads and cycad foliage).3 WHAT TO LOOK OUT FOR The adult female cycad aulacaspis scale has a white cover (scale), 1.2–1.6 mm long, ENVIRONMENTAL variable in shape and sometimes translucent enough to see the orange insect with its IMPACTS OVERSEAS orange eggs beneath. The scale of the male is white and elongate, 0.5–0.6 mm long. Photo: Jeffrey W. Lotz, Florida Department of Agriculture and Consumer Services, The cycad aulacaspis scale has decimated Bugwood.org | CC BY 3.0 cycads on Guam since it appeared there in 2003. The affected species, Cycas micronesica, was once the most common cause cycad extinctions around the world, Taiwan, 100,000 cycads were destroyed tree on Guam, but was listed by the IUCN 7 including in India10 and Indonesia11. The by an outbreak of the scale . It is difficult as endangered in 2006 following attacks continuous removal of plant sap by the to control in cultivation because of high by three insect pests, of which this scale scale depletes cycads of carbohydrates4,9. -

Chemical Element Concentrations of Cycad Leaves: Do We Know Enough?
horticulturae Review Chemical Element Concentrations of Cycad Leaves: Do We Know Enough? Benjamin E. Deloso 1 , Murukesan V. Krishnapillai 2 , Ulysses F. Ferreras 3, Anders J. Lindström 4, Michael Calonje 5 and Thomas E. Marler 6,* 1 College of Natural and Applied Sciences, University of Guam, Mangilao, GU 96923, USA; [email protected] 2 Cooperative Research and Extension, Yap Campus, College of Micronesia-FSM, Colonia, Yap 96943, Micronesia; [email protected] 3 Philippine Native Plants Conservation Society Inc., Ninoy Aquino Parks and Wildlife Center, Quezon City 1101, Philippines; [email protected] 4 Plant Collections Department, Nong Nooch Tropical Botanical Garden, 34/1 Sukhumvit Highway, Najomtien, Sattahip, Chonburi 20250, Thailand; [email protected] 5 Montgomery Botanical Center, 11901 Old Cutler Road, Coral Gables, FL 33156, USA; [email protected] 6 Western Pacific Tropical Research Center, University of Guam, Mangilao, GU 96923, USA * Correspondence: [email protected] Received: 13 October 2020; Accepted: 16 November 2020; Published: 19 November 2020 Abstract: The literature containing which chemical elements are found in cycad leaves was reviewed to determine the range in values of concentrations reported for essential and beneficial elements. We found 46 of the 358 described cycad species had at least one element reported to date. The only genus that was missing from the data was Microcycas. Many of the species reports contained concentrations of one to several macronutrients and no other elements. The cycad leaves contained greater nitrogen and phosphorus concentrations than the reported means for plants throughout the world. Magnesium was identified as the macronutrient that has been least studied. -

Bush Foods and Fibres
Australian Plants Society NORTH SHORE GROUP Ku-ring-gai Wildflower Garden Bush foods and fibres • Plant-based bush foods, medicines and poisons can come from nectar, flowers, fruit, leaves, bark, stems, sap and roots. • Plants provide fibres and materials for making many items including clothes, cords, musical instruments, shelters, tools, toys and weapons. • A fruit is the seed-bearing structure of a plant. • Do not eat fruits that you do not know to be safe to eat. Allergic reactions or other adverse reactions could occur. • We acknowledge the Traditional Custodians of this land and pay our respects to the Elders both past, present and future for they hold the memories, traditions, culture and hope of their people. Plants as food: many native plants must be processed before they are safe to eat. Flowers, nectar, pollen, Sugars, vitamins, honey, lerps (psyllid tents) minerals, starches, manna (e.g. Ribbon Gum proteins & other nutrients Eucalyptus viminalis exudate), gum (e.g. Acacia lerp manna decurrens) Fruit & seeds Staple foods Carbohydrates (sugars, starches, fibre), proteins, fats, vitamins Leaves, stalks, roots, apical Staple foods Carbohydrates, protein, buds minerals Plants such as daisies, lilies, orchids and vines Tubers, rhyzomes were a source of starchy tubers known as Carbohydrate, fibre, yams. The yam daisy Microseris lanceolata protein, vitamins, (Asteraceae) was widespread in inland NSW minerals and other states. The native yam Dioscorea transversa grows north from Stanwell Tops into Qld and Northern Territory and can be eaten raw or roasted as can those of Trachymene incisa. 1 Plant Description of food Other notes Acacia Wattle seed is a rich source of iron, Saponins and tannins and other essential elements. -

Report and Recommendations on Cycad Aulacaspis Scale, Aulacaspis Yasumatsui Takagi (Hemiptera: Diaspididae)
IUCN/SSC Cycad Specialist Group – Subgroup on Invasive Pests Report and Recommendations on Cycad Aulacaspis Scale, Aulacaspis yasumatsui Takagi (Hemiptera: Diaspididae) 18 September 2005 Subgroup Members (Affiliated Institution & Location) • William Tang, Subgroup Leader (USDA-APHIS-PPQ, Miami, FL, USA) • Dr. John Donaldson, CSG Chair (South African National Biodiversity Institute & Kirstenbosch National Botanical Garden, Cape Town, South Africa) • Jody Haynes (Montgomery Botanical Center, Miami, FL, USA)1 • Dr. Irene Terry (Department of Biology, University of Utah, Salt Lake City, UT, USA) Consultants • Dr. Anne Brooke (Guam National Wildlife Refuge, Dededo, Guam) • Michael Davenport (Fairchild Tropical Botanic Garden, Miami, FL, USA) • Dr. Thomas Marler (College of Natural & Applied Sciences - AES, University of Guam, Mangilao, Guam) • Christine Wiese (Montgomery Botanical Center, Miami, FL, USA) Introduction The IUCN/SSC Cycad Specialist Group – Subgroup on Invasive Pests was formed in June 2005 to address the emerging threat to wild cycad populations from the artificial spread of insect pests and pathogens of cycads. Recently, an aggressive pest on cycads, the cycad aulacaspis scale (CAS)— Aulacaspis yasumatsui Takagi (Hemiptera: Diaspididae)—has spread through human activity and commerce to the point where two species of cycads face imminent extinction in the wild. Given its mission of cycad conservation, we believe the CSG should clearly focus its attention on mitigating the impact of CAS on wild cycad populations and cultivated cycad collections of conservation importance (e.g., Montgomery Botanical Center). The control of CAS in home gardens, commercial nurseries, and city landscapes is outside the scope of this report and is a topic covered in various online resources (see www.montgomerybotanical.org/Pages/CASlinks.htm). -

Complexity and Variation in the Effects of Low-Severity Fires on Forest Biota
University of Montana ScholarWorks at University of Montana Graduate Student Theses, Dissertations, & Professional Papers Graduate School 2003 Complexity and variation in the effects of low-severity fires on forest biota Karen Christine Short The University of Montana Follow this and additional works at: https://scholarworks.umt.edu/etd Let us know how access to this document benefits ou.y Recommended Citation Short, Karen Christine, "Complexity and variation in the effects of low-severity fires on forest biota" (2003). Graduate Student Theses, Dissertations, & Professional Papers. 9463. https://scholarworks.umt.edu/etd/9463 This Dissertation is brought to you for free and open access by the Graduate School at ScholarWorks at University of Montana. It has been accepted for inclusion in Graduate Student Theses, Dissertations, & Professional Papers by an authorized administrator of ScholarWorks at University of Montana. For more information, please contact [email protected]. Maureen and Mike MANSFIELD LIBRARY The University of Montana Permission is granted by the author to reproduce this material in its entirety, provided that this material is used for scholarly purposes and is properly cited in published works and reports. **Please check "Yes" or "No" and provide signature** ,/ Yes, I grant permission U7 ______ No, I do not grant permission Author's Signature: Date: <£/£-/< Any copying for commercial purposes or financial gain may be undertaken only with the author's explicit consent. 8/98 Reproduced with permission of the copyright owner. Further reproduction prohibited without permission. Reproduced with permission of the copyright owner. Further reproduction prohibited without permission. COMPLEXITY AND VARIATION IN THE EFFECTS OF LOW-SEVERITY FIRES ON FOREST BIOTA by Karen C. -

Cycad Forensics: Tracing the Origin of Poached Cycads Using Stable Isotopes, Trace Element Concentrations and Radiocarbon Dating Techniques
Cycad forensics: Tracing the origin of poached cycads using stable isotopes, trace element concentrations and radiocarbon dating techniques by Kirsten Retief Supervisors: Dr Adam West (UCT) and Ms Michele Pfab (SANBI) Submitted in partial fulfillment of the requirements for the degree of Masters of Science in Conservation Biology 5 June 2013 Percy FitzPatrick Institution of African Ornithology, UniversityDepartment of Biologicalof Cape Sciences Town University of Cape Town, Rondebosch Cape Town South Africa 7701 i The copyright of this thesis vests in the author. No quotation from it or information derived from it is to be published without full acknowledgement of the source. The thesis is to be used for private study or non- commercial research purposes only. Published by the University of Cape Town (UCT) in terms of the non-exclusive license granted to UCT by the author. University of Cape Town Table of Contents Acknowledgements iii Plagiarism declaration iv Abstract v Chapter 1: Status of cycads and background to developing a forensic technique 1 1. Why are cycads threatened? 2 2. Importance of cycads 4 3. Current conservation strategies 5 4. Stable isotopes in forensic science 7 5. Trace element concentrations 15 6. Principles for using isotopes as a tracer 15 7. Radiocarbon dating 16 8. Cycad life history, anatomy and age of tissues 18 9. Recapitulation 22 Chapter 2: Applying stable isotope and radiocarbon dating techniques to cycads 23 1. Introduction 24 2. Methods 26 2.1 Sampling selection and sites 26 2.2 Sampling techniques 30 2.3 Processing samples 35 2.4 Cellulose extraction 37 2.5 Oxygen and sulphur stable isotopes 37 2.6 CarbonUniversity and nitrogen stable of isotopes Cape Town 38 2.7 Strontium, lead and elemental concentration analysis 39 2.8 Radiocarbon dating 41 2.9 Data analysis 42 3. -

1 REQUEST for STATEMENTS of INTEREST N40192-21-2-8003 PROJECT to BE INITIATED in FISCAL YEAR 2021 Project Title: CYCAD MONITORIN
REQUEST FOR STATEMENTS OF INTEREST N40192-21-2-8003 PROJECT TO BE INITIATED IN FISCAL YEAR 2021 Project Title: CYCAD MONITORING AT ANDERSEN AIR FORCE BASE (AAFB) AND TINIAN MILITARY LEASE AREA (MLA) Responses to this Request for Statements of Interest will be used to identify potential projects to be funded by the Department of the Navy (DoN) in support of monitoring the Cycas micronesica plants currently at Andersen Air Force Base (AAFB), Guam and the Tinian Military Lease Area (MLA). Approximately $219,491 is expected to be available to support this program (contingent upon availability of funds). The Department of Navy’s obligation to pay or reimburse any costs hereunder is subject to the availability of appropriated funds and limited by funds obligated and nothing in this Agreement will be interpreted to require obligations or payments by the Federal Government in violation of the Anti-Deficiency Act, 31 U.S.C. §1341. Thus, funds have not yet been appropriated for this project and there is considerable uncertainty regarding the level of available funding for FY2021. Background Fadang or Micronesian cycad (Cycas micronesica), an endemic plant found on the islands of Guam and Rota, was the dominant plant and the most abundant ‘tree’ in Guam’s forests in 2000. The invasion of the cycad scale Aulacaspis yasumatsui in 2003 and the butterfly Luthrodes pandava (formerly known as Chilades pandava) in 2005 initiated an epidemic mortality of plant populations such that C. micronesica was Red- listed by the IUCN as endangered in 2006 and listed as threatened under the U.S. -

Brisbane Native Plants by Suburb
INDEX - BRISBANE SUBURBS SPECIES LIST Acacia Ridge. ...........15 Chelmer ...................14 Hamilton. .................10 Mayne. .................25 Pullenvale............... 22 Toowong ....................46 Albion .......................25 Chermside West .11 Hawthorne................. 7 McDowall. ..............6 Torwood .....................47 Alderley ....................45 Clayfield ..................14 Heathwood.... 34. Meeandah.............. 2 Queensport ............32 Trinder Park ...............32 Algester.................... 15 Coopers Plains........32 Hemmant. .................32 Merthyr .................7 Annerley ...................32 Coorparoo ................3 Hendra. .................10 Middle Park .........19 Rainworth. ..............47 Underwood. ................41 Anstead ....................17 Corinda. ..................14 Herston ....................5 Milton ...................46 Ransome. ................32 Upper Brookfield .......23 Archerfield ...............32 Highgate Hill. ........43 Mitchelton ...........45 Red Hill.................... 43 Upper Mt gravatt. .......15 Ascot. .......................36 Darra .......................33 Hill End ..................45 Moggill. .................20 Richlands ................34 Ashgrove. ................26 Deagon ....................2 Holland Park........... 3 Moorooka. ............32 River Hills................ 19 Virginia ........................31 Aspley ......................31 Doboy ......................2 Morningside. .........3 Robertson ................42 Auchenflower -

Rdna) Organisation
OPEN Heredity (2013) 111, 23–33 & 2013 Macmillan Publishers Limited All rights reserved 0018-067X/13 www.nature.com/hdy ORIGINAL ARTICLE Dancing together and separate again: gymnosperms exhibit frequent changes of fundamental 5S and 35S rRNA gene (rDNA) organisation S Garcia1 and A Kovarˇı´k2 In higher eukaryotes, the 5S rRNA genes occur in tandem units and are arranged either separately (S-type arrangement) or linked to other repeated genes, in most cases to rDNA locus encoding 18S–5.8S–26S genes (L-type arrangement). Here we used Southern blot hybridisation, PCR and sequencing approaches to analyse genomic organisation of rRNA genes in all large gymnosperm groups, including Coniferales, Ginkgoales, Gnetales and Cycadales. The data are provided for 27 species (21 genera). The 5S units linked to the 35S rDNA units occur in some but not all Gnetales, Coniferales and in Ginkgo (B30% of the species analysed), while the remaining exhibit separate organisation. The linked 5S rRNA genes may occur as single-copy insertions or as short tandems embedded in the 26S–18S rDNA intergenic spacer (IGS). The 5S transcript may be encoded by the same (Ginkgo, Ephedra) or opposite (Podocarpus) DNA strand as the 18S–5.8S–26S genes. In addition, pseudogenised 5S copies were also found in some IGS types. Both L- and S-type units have been largely homogenised across the genomes. Phylogenetic relationships based on the comparison of 5S coding sequences suggest that the 5S genes independently inserted IGS at least three times in the course of gymnosperm evolution. Frequent transpositions and rearrangements of basic units indicate relatively relaxed selection pressures imposed on genomic organisation of 5S genes in plants. -

Garden Plants Poisonous to People
N NO V E M B E R 2 0 0 6 P R I M E F A C T 3 5 9 ( R E P L A C E S A G F A C T P 7 . 1 . 1 P O I S O N O U S P L A N T S I N T H E G A R D E N) Garden plants poisonous to people Annie Johnson Table 1. Toxicity rating for Tables 2−7. Weeds Project Officer Rating Toxicity Stephen Johnson Mildly toxic. Mild symptoms may occur if large * Weed Ecologist quantities are eaten. Toxic. Causes discomfort and irritation but not Weeds Unit, Biosecurity Compliance and Mine ** Safety, Orange dangerous to life. Highly toxic. Capable of causing serious illness *** or death. Introduction There are a range of garden plants that are considered poisonous. Poisonings and deaths from garden plants Poisoning are rare as most poisonous plants taste unpleasant Poisoning from plants may occur from ingesting, and are seldom swallowed (see toxicity). However, it is inhalation or direct contact. best to know which plants are potentially toxic. Symptoms from ingestion include gastroenteritis, It is important to remember that small children are diarrhoea, vomiting, nervous symptoms and in serious often at risk from coloured berries, petals and leaves cases, respiratory and cardiac distress. Poisoning that look succulent. This does not mean that all these by inhalation of pollen, dust or fumes from burning poisonous plants should be avoided or removed from plants can cause symptoms similar to hay fever or the garden. It is best to teach children never to eat asthma.