Crystallization of Kainite from Solutions in Syste
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Mineralogy of the Martian Surface
EA42CH14-Ehlmann ARI 30 April 2014 7:21 Mineralogy of the Martian Surface Bethany L. Ehlmann1,2 and Christopher S. Edwards1 1Division of Geological & Planetary Sciences, California Institute of Technology, Pasadena, California 91125; email: [email protected], [email protected] 2Jet Propulsion Laboratory, California Institute of Technology, Pasadena, California 91109 Annu. Rev. Earth Planet. Sci. 2014. 42:291–315 Keywords First published online as a Review in Advance on Mars, composition, mineralogy, infrared spectroscopy, igneous processes, February 21, 2014 aqueous alteration The Annual Review of Earth and Planetary Sciences is online at earth.annualreviews.org Abstract This article’s doi: The past fifteen years of orbital infrared spectroscopy and in situ exploration 10.1146/annurev-earth-060313-055024 have led to a new understanding of the composition and history of Mars. Copyright c 2014 by Annual Reviews. Globally, Mars has a basaltic upper crust with regionally variable quanti- by California Institute of Technology on 06/09/14. For personal use only. All rights reserved ties of plagioclase, pyroxene, and olivine associated with distinctive terrains. Enrichments in olivine (>20%) are found around the largest basins and Annu. Rev. Earth Planet. Sci. 2014.42:291-315. Downloaded from www.annualreviews.org within late Noachian–early Hesperian lavas. Alkali volcanics are also locally present, pointing to regional differences in igneous processes. Many ma- terials from ancient Mars bear the mineralogic fingerprints of interaction with water. Clay minerals, found in exposures of Noachian crust across the globe, preserve widespread evidence for early weathering, hydrothermal, and diagenetic aqueous environments. Noachian and Hesperian sediments include paleolake deposits with clays, carbonates, sulfates, and chlorides that are more localized in extent. -

S40645-019-0306-X.Pdf
Isaji et al. Progress in Earth and Planetary Science (2019) 6:60 Progress in Earth and https://doi.org/10.1186/s40645-019-0306-x Planetary Science RESEARCH ARTICLE Open Access Biomarker records and mineral compositions of the Messinian halite and K–Mg salts from Sicily Yuta Isaji1* , Toshihiro Yoshimura1, Junichiro Kuroda2, Yusuke Tamenori3, Francisco J. Jiménez-Espejo1,4, Stefano Lugli5, Vinicio Manzi6, Marco Roveri6, Hodaka Kawahata2 and Naohiko Ohkouchi1 Abstract The evaporites of the Realmonte salt mine (Sicily, Italy) are important archives recording the most extreme conditions of the Messinian Salinity Crisis (MSC). However, geochemical approach on these evaporitic sequences is scarce and little is known on the response of the biological community to drastically elevating salinity. In the present work, we investigated the depositional environments and the biological community of the shale–anhydrite–halite triplets and the K–Mg salt layer deposited during the peak of the MSC. Both hopanes and steranes are detected in the shale–anhydrite–halite triplets, suggesting the presence of eukaryotes and bacteria throughout their deposition. The K–Mg salt layer is composed of primary halites, diagenetic leonite, and primary and/or secondary kainite, which are interpreted to have precipitated from density-stratified water column with the halite-precipitating brine at the surface and the brine- precipitating K–Mg salts at the bottom. The presence of hopanes and a trace amount of steranes implicates that eukaryotes and bacteria were able to survive in the surface halite-precipitating brine even during the most extreme condition of the MSC. Keywords: Messinian Salinity Crisis, Evaporites, Kainite, μ-XRF, Biomarker Introduction hypersaline condition between 5.60 and 5.55 Ma (Manzi The Messinian Salinity Crisis (MSC) is one of the most et al. -

United States Patent (11) 3,615,174
United States Patent (11) 3,615,174 72 Inventor William J. Lewis 3,342,548 9/1967 Macey....... A. 2319 X South Ogden, Utah 3,432,031 3/1969 Ferris........................... 209/166 X 21 Appl. No. 740,886 FOREIGN PATENTS 22, Filed June 28, 1968 45 Patented Oct. 26, 1971 1,075,166 4f1954 France ......................... 209/66 (73) Assignee NL Industries, Inc. OTHER REFERENCES New York, N.Y. Chem. Abst., Vol. 53, 1959, 9587e I & EC, Vol. 56, 7, Jy '64, 61 & 62. Primary Examiner-Frank W. Lutter 54 PROCESSFOR THE SELECTIVE RECOVERY OF Assistant Examiner-Robert Halper POTASSUMAND MAGNESUMWALUES FROM Attorney-Ward, McElhannon, Brooks & Fitzpatrick AQUEOUSSALT SOLUTIONS CONTAINING THE SAME 11 Claims, 4 Drawing Figs. ABSTRACT: Kainite immersed in brine in equilibrium con 52) U.S. Cl........................................................ 23138, verted to carnalite by cooling to about 10 C. or under. Car 209/11, 209/166,23191, 22/121 nallite so obtained purified by cold flotation. Purified carnal (5) Int. Cl......................................................... B03b 1100, lite water leached to yield magnesium chloride brine and B03d 1102, C01f 5126 potassium chloride salt. Latter optionally converted to potas 50 Field of Search............................................ 209/166,3, sium sulfate by reaction with kainite, or by reacting the carnal 10, 11; 23.19, 38, 121 lite with kainite. Naturally occurring brine concentrated to precipitate principally sodium chloride, mother liquor warm 56 References Cited concentrated to precipitate kainite, cooled under mother UNITED STATES PATENTS liquor for conversion to carnallite. A crude kainite fraction 2,479,001 8/1949 Burke........................... 23.191 purified by warm flotation and a crude carnallite fraction pu 2,689,649 9, 1954 Atwood.... -

The Mineralogy of the British Permian Evaporites
460 The mineralogy of the British Permian evaporites By F. H. STEWART Department of Geology, University of Edinburgh Summary. The occurrence of the minerals is reviewed. Consideration of their genesis leads to a tentative list of primary minerals, and of early diagenetie, later diagenetic and geothermal metamorphic, and late near-surface changes. N 1943 Professor Tilley recorded the occurrence of po]yhalite in the I rocks of the D'Arcy Exploration Company's E.2 borehole at Aislaby, near Whitby; this was the first published record of a potassium-bearing salt in British evaporites. The occurrence of rocks containing sylvine (Lees and Taitt, 1946) prompted further exploration by Imperial Chemical Industries and Fisons Ltd. after the war, and this showed the presence of large deposits of potassium ores in the Whitby region. There is now a considerable literature related to these, and it seems timely to give a short review of the mineralogy of the British Permian evaporites. The writer is most grateful to Fisons Ltd. who have kindly allowed him to use unpublished information from his study of their boreholes F.1 at Robin Hood's Bay, F.2 at Staintondale, F.3 at Little Beck, and F.4 at Hawsker, in the Whitby Scarborough district. The position of these and the other boreholes mentioned in this paper can be found in the map of Dunham (1960). Dist~'ibution of the minerals The evaporites occur in two principal areas east and west of the Pennine Hills. In the western area (the Vale of Eden, west Cumberland, and the Furness district of Lancashire), where four evaporite beds have been recognized (Hollingworth, 1942), the mineralogy is relatively simple; carbonates, anhydrite, and gypsum are the main constituents. -

Mechanism and Kinetics of Dehydration of Epsomite Crystals Formed in the Presence of Organic Additives
J. Phys. Chem. B 2007, 111, 41-52 41 Mechanism and Kinetics of Dehydration of Epsomite Crystals Formed in the Presence of Organic Additives Encarnacio´n Ruiz-Agudo,† J. Daniel Martı´n-Ramos,‡ and Carlos Rodriguez-Navarro* Departamento de Mineralogı´a y Petrologı´a, UniVersidad de Granada, FuentenueVa s/n, 18002 Granada, Spain ReceiVed: July 14, 2006; In Final Form: October 10, 2006 The thermal dehydration of epsomite (MgSO4‚7H2O) crystals grown in the presence and absence of organic additives (phosphonates, carboxylic acids, and polyacrylic acid derivatives) was studied by means of thermogravimetry (TG), differential scanning calorimetry (DSC), X-ray thermodiffraction (XRTD), and environmental scanning electron microscopy (ESEM). In situ XRTD analyses (in air, 30% relative humidity) show an epsomite f hexahydrite (MgSO4‚6H2O) transition at 25-38 °C, followed by formation of amorphous phase(s) at T > 43-48 °C, and MgSO4 crystallization at ∼300 °C. Kinetic parameters (ER and A) were determined for the main dehydration step (25-160 °C), which corresponds to a MgSO4‚7H2O f MgSO4‚ H2O transition, by applying two isoconversional methods to nonisothermal TG data obtained at different heating rates (â ) 1, 3, and 5 K‚min-1). In situ, hot-stage ESEM observations of the thermal dehydration of epsomite crystals are consistent with the nonisothermal kinetic study and, along with XRTD results, allow us to propose a dehydration mechanism which includes an early nucleation and growth event, followed by the advancement of the reaction interface (3D phase boundary reaction). Both ER and A values increase in the presence of the most effective crystallization inhibitors tested. -

Stability of Magnesium Sulfate Minerals in Martian Environments
Lunar and Planetary Science XXXVI (2005) 2290.pdf STABILITY OF MAGNESIUM SULFATE MINERALS IN MARTIAN ENVIRONMENTS. G.M. Marion1 and J.S. Kargel2, 1Desert Research Institute, 2215 Raggio Parkway, Reno, NV 89512, [email protected], 2U.S. Geological Survey, 2255 N. Gemini Dr., Flagstaff, AZ 86001, [email protected]. Introduction: Viking Lander, Pathfinder, and –3.6°C for a pure MgSO4-H2O system. In this system, Mars Exploration Rover missions to Mars have found the lower hydrates, hexahydrite and kieserite, only abundant sulfur in surface soils and rocks, and the best form at higher temperatures (48°C and 69°C, respec- indications are that magnesium sulfates are among the tively). key hosts [1-6]. At Meridiani Planum, MgSO4 salts The presence of other constituents in ternary (or constitute 15 to 40 wt.% of sedimentary rocks [3-5,7]. higher) systems can lower the temperatures at which Additional S is hosted by gypsum and jarosite. Reflec- lower hydrates are stable. For example, hexahydrite is tance and thermal emission spectroscopy is consistent stable at 25°C in a MgSO4-MgCl2-H2O system at 3.8 with the presence of kieserite (MgSO4•H2O) and ep- m MgCl2 [12]. This requires high chloride concentra- somite (MgSO4•7H2O) [3]. Theoretically, the tions that may occur in Martian environments that dodecahydrate (MgSO4•12H2O) should also have have previously undergone extensive sulfate precipita- precipitated [8]. tion and chloride concentration due to extreme freez- We first examine theoretically which MgSO4 min- ing or desiccating conditions [3-5]. erals should have precipitated on Mars, and then how Based on the Rover mission to Meridiani Planum, dehydration might have altered these minerals. -

Gases in Evaporites: Part 2 of 3: Nature, Distribution and Sources
www.saltworkconsultants.com Salty MattersJohn Warren - Wed., November 30, 2016 Gases in Evaporites: Part 2 of 3: Nature, distribution and sources This, the second of three articles on gases held within salt de- older and younger sets of gas analyses conducted over the years posits, focuses on the types of gases found in salt and their ori- in various salt deposits are not necessarily directly comparable. gins. The first article (Salty Matters October 31, 2016) dealt with Raman micro-spectroscopy is a modern, non-destructive meth- the impacts of intersecting gassy salt pockets during mining or od for investigating the unique content of a single inclusion in drilling operations. The third will discuss the distribution of the a salt crystal. There is a significant difference in terms of what is various gases with respect to broad patterns of salt mass shape measured in analysing gas content seeping from a fissure in a salt and structure (bedded, halokinetic and fractured) mass or if comparisons are made with conventional wet-chem- ical methods which were the pre Raman-microscopy method What’s the gas? that is sometimes still used. Wet chemical methods require sam- Gases held in evaporites are typically mixtures of varying propor- ple destruction, via crushing and subsequent dissolution, prior to tions of nitrogen, methane, carbon dioxide, hydrogen, hydrogen analysis. This can lead to the escape of a variable proportion of sulphide, as well as brines and minor amounts of Units dominated by Units dominated by Units with more or other gases such as argon inclusions from the N-O inclusions from less equal numbers CH -H -H S groups of both groups and various short chain and N2 groups 4 2 2 hydrocarbons (Table 2). -

High Temperature Sulfate Minerals Forming on the Burning Coal Dumps from Upper Silesia, Poland
minerals Article High Temperature Sulfate Minerals Forming on the Burning Coal Dumps from Upper Silesia, Poland Jan Parafiniuk * and Rafał Siuda Faculty of Geology, University of Warsaw, Zwirki˙ i Wigury 93, 02-089 Warszawa, Poland; [email protected] * Correspondence: j.parafi[email protected] Abstract: The subject of this work is the assemblage of anhydrous sulfate minerals formed on burning coal-heaps. Three burning heaps located in the Upper Silesian coal basin in Czerwionka-Leszczyny, Radlin and Rydułtowy near Rybnik were selected for the research. The occurrence of godovikovite, millosevichite, steklite and an unnamed MgSO4, sometimes accompanied by subordinate admixtures of mikasaite, sabieite, efremovite, langbeinite and aphthitalite has been recorded from these locations. Occasionally they form monomineral aggregates, but usually occur as mixtures practically impossible to separate. The minerals form microcrystalline masses with a characteristic vesicular structure resembling a solidified foam or pumice. The sulfates crystallize from hot fire gases, similar to high temperature volcanic exhalations. The gases transport volatile components from the center of the fire but their chemical compositions are not yet known. Their cooling in the near-surface part of the heap results in condensation from the vapors as viscous liquid mass, from which the investigated minerals then crystallize. Their crystallization temperatures can be estimated from direct measurements of the temperatures of sulfate accumulation in the burning dumps and studies of their thermal ◦ decomposition. Millosevichite and steklite crystallize in the temperature range of 510–650 C, MgSO4 Citation: Parafiniuk, J.; Siuda, R. forms at 510–600 ◦C and godovikovite in the slightly lower range of 280–450 (546) ◦C. -

Assessment of the Molecular Structure of the Borate Mineral Boracite
Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy 96 (2012) 946–951 Contents lists available at SciVerse ScienceDirect Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy journal homepage: www.elsevier.com/locate/saa Assessment of the molecular structure of the borate mineral boracite Mg3B7O13Cl using vibrational spectroscopy ⇑ Ray L. Frost a, , Yunfei Xi a, Ricardo Scholz b a School of Chemistry, Physics and Mechanical Engineering, Science and Engineering Faculty, Queensland University of Technology, G.P.O. Box 2434, Brisbane, Queensland 4001, Australia b Geology Department, School of Mines, Federal University of Ouro Preto, Campus Morro do Cruzeiro, Ouro Preto, MG 35400-00, Brazil highlights graphical abstract " Boracite is a magnesium borate mineral with formula: Mg3B7O13Cl. " The crystals belong to the orthorhombic – pyramidal crystal system. " The molecular structure of the mineral has been assessed. " Raman spectrum shows that some Cl anions have been replaced with OH units. article info abstract Article history: Boracite is a magnesium borate mineral with formula: Mg3B7O13Cl and occurs as blue green, colorless, Received 23 May 2012 gray, yellow to white crystals in the orthorhombic – pyramidal crystal system. An intense Raman band Received in revised form 2 July 2012 À1 at 1009 cm was assigned to the BO stretching vibration of the B7O13 units. Raman bands at 1121, Accepted 9 July 2012 1136, 1143 cmÀ1 are attributed to the in-plane bending vibrations of trigonal boron. Four sharp Raman Available online 13 August 2012 bands observed at 415, 494, 621 and 671 cmÀ1 are simply defined as trigonal and tetrahedral borate bending modes. The Raman spectrum clearly shows intense Raman bands at 3405 and 3494 cmÀ1, thus Keywords: indicating that some Cl anions have been replaced with OH units. -
![The Paragenesis of Sylvine, Carnallite, Polyhalite, and ]Cieserite in Eskdale Borings Nos](https://docslib.b-cdn.net/cover/6717/the-paragenesis-of-sylvine-carnallite-polyhalite-and-cieserite-in-eskdale-borings-nos-1696717.webp)
The Paragenesis of Sylvine, Carnallite, Polyhalite, and ]Cieserite in Eskdale Borings Nos
667 The paragenesis of sylvine, carnallite, polyhalite, and ]cieserite in Eskdale borings nos. 3, 4, and 6, north-east Yorkshire. (With Plate XXV.) G. ARMSTRO~O, B.Se., F.G.S., K. C. DUNHAM, D.Sc., S.D., F.G.S., C. 0. HAI~VEY, B.Sc., A.R.C.S., F.R.I.C., P. A. SABINe, Ph.D., A.R.C.S., F.G.S., and W. F. WATERS, B.Sc., F.R.I.C. Geological Survey of Great Britain. [Read March 8, 1951.] INTRODUCTION. HE confirmation of the presence of potassium salts in the Permian T evaporites beneath the Whitby district, first found in the D'Arey Exploration Co.'s Eskdale no. 2 (Aislaby) boring (G. M. Lees and A. H. Taitt, 1945), and the discovery by Imperial Chemical Industries in their Eskdale no. 3 (Sleights), no. 4 (Sneaton), and no. 6 (Upgang) borings that these salts exist in workable quality and quantity, has been recorded in a recent historic paper by Dr. A. Fleck (1950). The successful outcome of this boring campaign may well lead to the outstanding development of the present century in economic mineralogy in Britain. In the course of the exploration the Geological Survey has examined in detail the cores obtained, and every facility has been granted by Imperial Chemical Industries for the removal of specimens from the cores for preservation in the national collection at the Geological Museum. The Petrographical Department of the Geological Survey has, on its side, provided reports on the mineralogy of the cores. The present brief summary of these reports, with some additional chemical, X-ray, and petrographical data, is published by permission of the Director of the Geological Survey and with the approval of Imperial Chemical Industries, Limited. -

Congolite and Trembathite from the Kłodawa Salt Mine, Central Poland: Records of the Thermal History of the Parental Salt Dome
1387 The Canadian Mineralogist Vol. 50, pp. 1387-1399 (2012) DOI : 10.3749/canmin.50.5.1387 CONGOLITE AND TREMBATHITE FROM THE KŁODAWA SALT MINE, CENTRAL POLAND: RECORDS OF THE THERMAL HISTORY OF THE PARENTAL SALT DOME JACEK WACHOWIAK§ AGH – University of Science and Technology, Department of Economic and Mining Geology; al. Mickiewicza 30, 30-059 Kraków, Poland ADAM PIECZKA AGH – University of Science and Technology, Department of Mineralogy, Petrography and Geochemistry; al. Mickiewicza 30, 30-059 Kraków, Poland ABSTRACT Pseudocubic crystals of (Fe,Mg,Mn)3B7O13Cl borate, ≤ 1.2 mm in size and yellowish through pale-violet to pale-violet- brown and brownish in color, were found in the Kłodawa salt dome, located within the Mid-Polish Trough (central Poland), an axial part of the Polish basin belonging to a system of Permian-Mesozoic epicontinental basins of Western and Central Europe. The crystals occur in adjacent parts of the Underlying Halite and Youngest Halite units bordering the Anhydrite Pegmatite unit around the PZ–3/PZ–4 (Leine/Aller) boundary. The internal texture of the crystals reflects phase-transitions from rhombohedral congolite (space-group symmetry R3c) through orthorhombic ericaite (Pca21) to a cubic, high-temperature, mainly Fe-dominant analogue of boracite (F43c), indicating formation of the phases under increasing temperature. Currently, the parts of the crystals showing orthorhombic and cubic morphology generally represent congolite and, much less frequently, trembathite paramorphs after higher-temperature structural varieties of (Fe,Mg,Mn)3B7O13Cl and (Mg,Fe,Mn)3B7O13Cl, which are unstable at room- temperature. The phase-transitions R3c → Pca21 and Pca21 → F43c recorded in the morphology of the growing crystals took place at temperatures of 230–250 °C and 310–315 °C, respectively, whereas the total range of (Fe,Mg,Mn)3B7O13Cl borate crystallization was determined to be ca. -
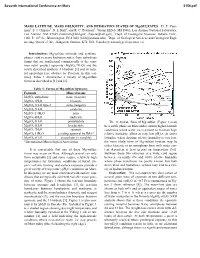
MARS LATITUDE, MARS OBLIQUITY, and HYDRATION STATES of Mg-SULFATES
Seventh International Conference on Mars 3156.pdf MARS LATITUDE, MARS OBLIQUITY, AND HYDRATION STATES OF Mg-SULFATES. D. T. Vani- man1, S. J. Chipera1, D. L Bish2, and R. C. Peterson3, 1Group EES-6, MS D462, Los Alamos National Laboratory, Los Alamos, NM 87545 ([email protected]; [email protected]), 2Dept. of Geological Sciences, Indiana Univ., 1001 E. 10th St., Bloomington, IN 47405 ([email protected]), 3Dept. of Geological Sciences and Geological Engi- neering, Queen’s Univ., Kingston, Ontario, K7L 3N6, Canada ([email protected]). Introduction: Mg-sulfate minerals and synthetic phases exist in many hydration states, from anhydrous forms that are synthesized commercially to the com- mon retail product epsomite (MgSO4·7H2O) and the newly described synthetic 11-hydrate [1] and its natu- ral equivalent [see abstract by Peterson in this vol- ume]. Table 1 summarizes a variety of Mg-sulfate forms as described in [1] and [2]. Table 1: Forms of Mg-sulfate hydrates Formula Mineral name MgSO4 anhydrous none (reagent) MgSO4·1H2O kieserite MgSO4·1H2O type 2 none (reagent) MgSO4·2H2O sanderite MgSO4·2.4H2O none MgSO ·4H O starkeyite 4 2 MgSO4·5H2O pentahydrite The 11-hydrate form of Mg-sulfate (Figure 1) may MgSO4·6H2O hexahydrite be a stable phase on Mars under current higher-latitude MgSO4·7H2O epsomite conditions where water ice is present to maintain high MgSO4·11H2O pending approval by IMA* relative humidity, albeit at very low pH2O. At lower MgSO4·nH2O amorphous (n variable) latitudes, where daytime relative humidity is very low, *International Mineralogical Association the more likely form of Mg-sulfate hydrate may be either kieserite or an amorphous form with water con- It is conceivable that any of these Mg-sulfate tent dependent at least in part on temperature [5,6].