Red-Footed Booby Helper at Great Frigatebird Nests
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Conservation Problems on Tristan Da Cunha Byj
28 Oryx Conservation Problems on Tristan da Cunha ByJ. H. Flint The author spent two years, 1963-65, as schoolmaster on Tristan da Cunha, during which he spent four weeks on Nightingale Island. On the main island he found that bird stocks were being depleted and the islanders taking too many eggs and young; on Nightingale, however, where there are over two million pairs of great shearwaters, the harvest of these birds could be greater. Inaccessible Island, which like Nightingale, is without cats, dogs or rats, should be declared a wildlife sanctuary. Tl^HEN the first permanent settlers came to Tristan da Cunha in " the early years of the nineteenth century they found an island rich in bird and sea mammal life. "The mountains are covered with Albatross Mellahs Petrels Seahens, etc.," wrote Jonathan Lambert in 1811, and Midshipman Greene, who stayed on the island in 1816, recorded in his diary "Sea Elephants herding together in immense numbers." Today the picture is greatly changed. A century and a half of human habitation has drastically reduced the larger, edible species, and the accidental introduction of rats from a shipwreck in 1882 accelerated the birds' decline on the main island. Wood-cutting, grazing by domestic stock and, more recently, fumes from the volcano have destroyed much of the natural vegetation near the settlement, and two bird subspecies, a bunting and a flightless moorhen, have become extinct on the main island. Curiously, one is liable to see more birds on the day of arrival than in several weeks ashore. When I first saw Tristan from the decks of M.V. -

How Seabirds Plunge-Dive Without Injuries
How seabirds plunge-dive without injuries Brian Changa,1, Matthew Crosona,1, Lorian Strakerb,c,1, Sean Garta, Carla Doveb, John Gerwind, and Sunghwan Junga,2 aDepartment of Biomedical Engineering and Mechanics, Virginia Polytechnic Institute and State University, Blacksburg, VA 24061; bNational Museum of Natural History, Smithsonian Institution, Washington, DC 20560; cSetor de Ornitologia, Museu Nacional, Universidade Federal do Rio de Janeiro, São Cristóvão, Rio de Janeiro RJ 20940-040, Brazil; and dNorth Carolina Museum of Natural Sciences, Raleigh, NC 27601 Edited by David A. Weitz, Harvard University, Cambridge, MA, and approved August 30, 2016 (received for review May 27, 2016) In nature, several seabirds (e.g., gannets and boobies) dive into wa- From a mechanics standpoint, an axial force acting on a slender ter at up to 24 m/s as a hunting mechanism; furthermore, gannets body may lead to mechanical failure on the body, otherwise known and boobies have a slender neck, which is potentially the weakest as buckling. Therefore, under compressive loads, the neck is po- part of the body under compression during high-speed impact. In tentially the weakest part of the northern gannet due to its long this work, we investigate the stability of the bird’s neck during and slender geometry. Still, northern gannets impact the water at plunge-diving by understanding the interaction between the fluid up to 24 m/s without injuries (18) (see SI Appendix, Table S1 for forces acting on the head and the flexibility of the neck. First, we estimated speeds). The only reported injuries from plunge-diving use a salvaged bird to identify plunge-diving phases. -

MAGNIFICENT FRIGATEBIRD Fregata Magnificens
PALM BEACH DOLPHIN PROJECT FACT SHEET The Taras Oceanographic Foundation 5905 Stonewood Court - Jupiter, FL 33458 - (561-762-6473) [email protected] MAGNIFICENT FRIGATEBIRD Fregata magnificens CLASS: Aves ORDER: Suliformes FAMILY: Fregatidae GENUS: Fregata SPECIES: magnificens A long-winged, fork-tailed bird of tropical oceans, the Magnificent Frigatebird is an agile flier that snatches food off the surface of the ocean and steals food from other birds. It breeds mostly south of the United States, but wanders northward along the coasts during nonbreeding season. Physical Appearance: Frigate birds are the only seabirds where the male and female look strikingly different. All have pre- dominantly black plumage, long, deeply forked tails and long hooked bills. Females have white underbellies and males have a distinctive red throat pouch, which they inflate during the breeding season to attract females Their wings are long and pointed and can span up to 2.3 meters (7.5 ft), the largest wing area to body weight ratio of any bird. These birds are about 35-45 inches ((89 to 114 cm) in length, and weight between 35 and 67 oz (1000-1900 g). The bones of frigate birds are markedly pneumatic (filled with air), making them very light and contribute only 5% to total body weight. The pectoral girdle (shoulder joint) is strong as its bones are fused. Habitat: Frigate birds are found across all tropical oceans. Breeding habitats include mangrove cays on coral reefs, and decidu- ous trees and bushes on dry islands. Feeding range while breeding includes shallow water within lagoons, coral reefs, and deep ocean out of sight of land. -

Breeding Biology of the Brown Noddy on Tern Island, Hawaii
Wilson Bull., 108(2), 1996, pp. 317-334 BREEDING BIOLOGY OF THE BROWN NODDY ON TERN ISLAND, HAWAII JENNIFER L. MEGYESI’ AND CURTICE R. GRIFFINS ABSTRACT.-we observed Brown Noddy (Anous stolidus pileatus) breeding phenology and population trends on Tern Island, French Frigate Shoals, Hawaii, from 1982 to 1992. Peaks of laying ranged from the first week in January to the first week in November; however, most laying occurred between March and September each year. Incubation length was 34.8 days (N = 19, SD = 0.6, range = 29-37 days). There were no differences in breeding pairs between the measurements of the first egg laid and successive eggs laid within a season. The proportion of light- and dark-colored chicks was 26% and 74%, respectively (N = 221) and differed from other Brown Noddy colonies studied in Atlantic and Pacific oceans. The length of time between clutches depended on whether the previous outcome was a failed clutch or a successfully fledged chick. Hatching, fledging, and reproductive success were significantly different between years. The subspecies (A. s. pihtus) differs in many aspects of its breeding biology from other colonies in the Atlantic and Pacific oceans, in regard to year-round occurrence at the colony, frequent renesting attempts, large egg size, proportion of light and dark colored chicks, and low reproductive success caused by in- clement weather and predation by Great Frigatebirds (Fregata minor). Received 31 Mar., 1995, accepted 5 Dec. 1995. The Brown Noddy (Anous stolidus) is the largest and most widely distributed of the tropical and subtropical tern species (Cramp 1985). -

Phylogenetic Patterns of Size and Shape of the Nasal Gland Depression in Phalacrocoracidae
PHYLOGENETIC PATTERNS OF SIZE AND SHAPE OF THE NASAL GLAND DEPRESSION IN PHALACROCORACIDAE DOUGLAS SIEGEL-CAUSEY Museumof NaturalHistory and Department of Systematicsand Ecology, University of Kansas, Lawrence, Kansas 66045-2454 USA ABSTRACT.--Nasalglands in Pelecaniformesare situatedwithin the orbit in closelyfitting depressions.Generally, the depressionsare bilobedand small,but in Phalacrocoracidaethey are more diversein shapeand size. Cormorants(Phalacrocoracinae) have small depressions typical of the order; shags(Leucocarboninae) have large, single-lobeddepressions that extend almost the entire length of the frontal. In all PhalacrocoracidaeI examined, shape of the nasalgland depressiondid not vary betweenfreshwater and marine populations.A general linear model detectedstrongly significant effectsof speciesidentity and gender on size of the gland depression.The effectof habitat on size was complexand was detectedonly as a higher-ordereffect. Age had no effecton size or shapeof the nasalgland depression.I believe that habitat and diet are proximateeffects. The ultimate factorthat determinessize and shape of the nasalgland within Phalacrocoracidaeis phylogenetichistory. Received 28 February1989, accepted1 August1989. THE FIRSTinvestigations of the nasal glands mon (e.g.Technau 1936, Zaks and Sokolova1961, of water birds indicated that theseglands were Thomson and Morley 1966), and only a few more developed in species living in marine studies have focused on the cranial structure habitats than in species living in freshwater associatedwith the nasal gland (Marpies 1932; habitats (Heinroth and Heinroth 1927, Marpies Bock 1958, 1963; Staaland 1967; Watson and Di- 1932). Schildmacher (1932), Technau (1936), and voky 1971; Lavery 1972). othersshowed that the degree of development Unlike most other birds, Pelecaniformes have among specieswas associatedwith habitat. Lat- nasal glands situated in depressionsfound in er experimental studies (reviewed by Holmes the anteromedialroof of the orbit (Siegel-Cau- and Phillips 1985) established the role of the sey 1988). -

P0529-P0540.Pdf
RESPONSES TO HIGH TEMPERATURE IN NESTLING DOUBLE-CRESTED AND PELAGIC CORMORANTS ROBERT C. L^$IEWSKI AND GREGORY K. SNYDER ADULTand nestlingcormorants are often subjectto overheatingfrom insolationat the nest. Their generallydark plumage,exposed nest sites, and reradiation from surroundingrocks aggravate the thermal stress. Young nestlingsmust be shieldedfrom the sun by their parents. Older nestlingsand adults compensatefor heat gain throughbehavioral adjust- mentsand modulationof evaporativecooling by pantingand gular flutter- ing. This study was undertakento examinesome of the responsesto high temperaturein nestlingsof two speciesof cormorants,the Double-crested Cormorant,Phalacrocorax auritus, and the PelagicCormorant, P. pelagicus. The evaporative cooling responsesin birds have been studied in some detail in recent years (see Bartholomewet al., 1962; Lasiewski et al., 1966; Bartholomewet al., 1968; Calder and Schmidt-Nielsen,1968, for more detailed discussions),although much still remains to be learned. MATERIALS AND •VIETI-IODS The nestling cormorantsused in this study (four Phalacrocoraxpelagicus and four P. auritus) were captured from nests on rocky islands off the northwest coast of Washington. As their dates of hatchingwere not known it was impossibleto provide exact ages. From comparisonsof feather developmentwith descriptionsin the literature (Bent, 1922; Palmer, 1962), we judged that the pe'lagicuschicks were approximately 5, 5, 6, and 6 weeksold, while'the auritus chickswere 3.5, 3.5, 4.5, and 6 weeksof age upon capture. The nestlingswere taken to the laboratoriesat Friday Harbor, Washington on the day of capture and housed in three 4' X 4' X 4' chicken wire cages. The cageswere equippedwith plywood platforms for the birds to sit on and coveredon top and two sides to shield birds from wind and rain. -

GREAT FRIGATEBIRD Fregata Minor
GREAT FRIGATEBIRD Fregata minor Other: ‘Iwa F.m. palmerstoni breeding visitor, indigenous Great Frigatebird is nearly a pantropical species, being absent only from the Atlantic Ocean north of the equator (King 1967, Harrison 1983, Marchant and Higgins 1990, Metz and Schreiber 2002). It is a common breeder at Johnston and Wake atolls (Amerson and Shelton 1976, Rauzon et al. 2008). In the Northwestern Islands it breeds in Mar-Oct, it roosts in large numbers on certain islets of the Southeastern Islands, and is a fairly common sight soaring over most islands (although less common over Hawai’i I) throughout the year. POBSP data indicate a slight depression in monthly counts during Nov-Feb, especially in the more northwestern of the islands; when not breeding, it appears to disperse widely throughout tropical and subtropical seas, with vagrants recorded N to California (CBRC 2007), and individuals banded at Kure recovered as far away as the Marshall and Philippine Is (Woodward 1972). Hadden (1941) records a large movement of birds from Midway toward Kure on 29 Dec 1938. A frigatebird, probably Great, is present in the fossil record of Ulupau Head, O'ahu, indicating presence in the islands for at least 200,000 years (James 1987). There was much confusion about the naming of frigatebirds in the 1800s. Early ornithologists in Hawaii (Isenbeck in Kittlitz 1834, Dole 1869, Stejneger 1888, Wilson and Evans 1899, Rothschild 1900) considered the Hawaiian population to be the same species as Ascension Island Frigatebird (F. aquila) of the c. Atlantic Ocean, although Cassin (1858) and Dole (1879) correctly assigned them to "palmerstoni" based on Gmelin's (1789) "Pelecanus palmerstoni" from Palmerston I in the Cook Is group. -
The Genus Sula in the Carolinas: an Overview of the Phenology And
h Gn Sl n th Crln: An Ovrv f th hnl nd trbtn f Gnnt nd b n th Sth Atlnt ht DAVID S. LEE and J. CHRISTOPHER HANEY Five of the eight recognized species of the genus Sula are known from the southeastern United States. Of these only the Northern Gannet (Sula bassana) occurs regularly in the Carolinas, but both the Masked Booby (S. dactylatra), formerly Blue- faced, and the Brown Booby (S. leucogaster) have been reported from North and South Carolina. Of the two remaining species, the Red-footed Booby (S. sula) is generally restricted to the Caribbean and disperses northward into the Florida Keys and Gulf of Mexico, whereas the Blue-footed Booby (S. nebouxii) is an eastern Pacific species with one accidental and astonishing record from south Padre Island, Texas (5 October 1976, photograph Amer. Birds 31:349-351). Generally the records for locally occurring Sula, excluding wintering Northern Gannets, are less than adequate as conclusive evidence of seasonal or geographical occurrence. Most problems result from confusing plumages of the various species and the general lack of experience of North American bird students with boobies. An additional problem is the fact that until very recently most ornithologists believed that boobies occurred off the south Atlantic states, outside Florida, only as rare accidentals, causing many records to be viewed with excessive caution and skepticism. Potter et al. (1980), for example, associated all records of boobies in the Carolinas with storms. In recent years few groups of birds have caused as many interpretive problems for the Carolina Bird Club's North Carolina Records Committee as have the Sula. -
![A Report on the Guano-Producing Birds of Peru [“Informe Sobre Aves Guaneras”]](https://docslib.b-cdn.net/cover/2754/a-report-on-the-guano-producing-birds-of-peru-informe-sobre-aves-guaneras-982754.webp)
A Report on the Guano-Producing Birds of Peru [“Informe Sobre Aves Guaneras”]
PACIFIC COOPERATIVE STUDIES UNIT UNIVERSITY OF HAWAI`I AT MĀNOA Dr. David C. Duffy, Unit Leader Department of Botany 3190 Maile Way, St. John #408 Honolulu, Hawai’i 96822 Technical Report 197 A report on the guano-producing birds of Peru [“Informe sobre Aves Guaneras”] July 2018* *Original manuscript completed1942 William Vogt1 with translation and notes by David Cameron Duffy2 1 Deceased Associate Director of the Division of Science and Education of the Office of the Coordinator in Inter-American Affairs. 2 Director, Pacific Cooperative Studies Unit, Department of Botany, University of Hawai‘i at Manoa Honolulu, Hawai‘i 96822, USA PCSU is a cooperative program between the University of Hawai`i and U.S. National Park Service, Cooperative Ecological Studies Unit. Organization Contact Information: Pacific Cooperative Studies Unit, Department of Botany, University of Hawai‘i at Manoa 3190 Maile Way, St. John 408, Honolulu, Hawai‘i 96822, USA Recommended Citation: Vogt, W. with translation and notes by D.C. Duffy. 2018. A report on the guano-producing birds of Peru. Pacific Cooperative Studies Unit Technical Report 197. University of Hawai‘i at Mānoa, Department of Botany. Honolulu, HI. 198 pages. Key words: El Niño, Peruvian Anchoveta (Engraulis ringens), Guanay Cormorant (Phalacrocorax bougainvillii), Peruvian Booby (Sula variegate), Peruvian Pelican (Pelecanus thagus), upwelling, bird ecology behavior nesting and breeding Place key words: Peru Translated from the surviving Spanish text: Vogt, W. 1942. Informe elevado a la Compañia Administradora del Guano par el ornitólogo americano, Señor William Vogt, a la terminación del contracto de tres años que con autorización del Supremo Gobierno celebrara con la Compañia, con el fin de que llevara a cabo estudios relativos a la mejor forma de protección de las aves guaneras y aumento de la produción de las aves guaneras. -

Nazca Booby Sula Granti and Brewster's Brown
VanderWerf et al.: Nazca and Brewster’s Brown Boobies in Hawaii 67 NAZCA BOOBY SULA GRANTI AND BREWSTER’S BROWN BOOBY SULA LEUCOGASTER BREWSTERI IN THE HAWAIIAN ISLANDS AND JOHNSTON AND PALMYRA ATOLLS ERIC A. VANDERWERF1, BRENDA L. BECKER2, JAAP EIJZENGA3 & HEATHER EIJZENGA4 1Pacific Rim Conservation, 3038 Oahu Avenue, Honolulu, Hawaii, 96822, USA ([email protected]) 2National Marine Fisheries Service, 1601 Kapiolani Boulevard, Suite 1110, Honolulu, Hawaii, 96814, USA 3Hawaii Division of Forestry and Wildlife, 2135 Makiki Heights Drive, Honolulu, Hawaii, 96822, USA 4Bishop Museum, 1525 Bernice Street, Honolulu, Hawaii, 96817, USA Received 14 August 2007, accepted 29 May 2008 SUMMARY VANDERWERF, E.A., BECKER, B.L., EIJZENGA, J. & EIJZENGA, H. 2008. Nazca Booby Sula granti and Brewster’s Brown Booby Sula leucogaster brewsteri in the Hawaiian Islands and Johnston and Palmyra atolls. Marine Ornithology 36: 67–71. Nazca Booby (Sula granti) and Brewster’s Brown Booby (S. leucogaster brewsteri) are tropical sulids that normally occur only in the eastern Pacific Ocean. In this paper, we report on recent observations of Nazca Booby and Brewster’s Brown Booby in the Hawaiian Islands, including the first apparent nesting records, and we summarize other occurrences of these taxa in the Hawaiian Islands and Johnston and Palmyra Atolls. Genetic research has shown significant population structure between Brown Boobies in the eastern and central Pacific, but little population structure in Masked Boobies (S. dactylatra), indicating that the Eastern Pacific Basin has served as a dispersal barrier in Brown Boobies but not in Masked Boobies. Recent observations of brown-headed male Brown Boobies from the central Pacific nesting on Isla San Benedicto near Mexico indicate that some eastward dispersal is now occurring. -
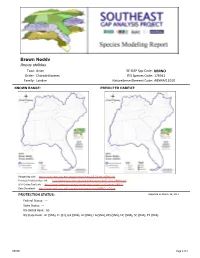
Brown Noddy Anous Stolidus Taxa: Avian SE-GAP Spp Code: Bbrno Order: Charadriiformes ITIS Species Code: 176941 Family: Laridae Natureserve Element Code: ABNNM11010
Brown Noddy Anous stolidus Taxa: Avian SE-GAP Spp Code: bBRNO Order: Charadriiformes ITIS Species Code: 176941 Family: Laridae NatureServe Element Code: ABNNM11010 KNOWN RANGE: PREDICTED HABITAT: P:\Proj1\SEGap P:\Proj1\SEGap Range Map Link: http://www.basic.ncsu.edu/segap/datazip/maps/SE_Range_bBRNO.pdf Predicted Habitat Map Link: http://www.basic.ncsu.edu/segap/datazip/maps/SE_Dist_bBRNO.pdf GAP Online Tool Link: http://www.gapserve.ncsu.edu/segap/segap/index2.php?species=bBRNO Data Download: http://www.basic.ncsu.edu/segap/datazip/region/vert/bBRNO_se00.zip PROTECTION STATUS: Reported on March 14, 2011 Federal Status: --- State Status: --- NS Global Rank: G5 NS State Rank: AL (SNA), FL (S1), GA (SNA), HI (SNR), LA (SNA), MS (SNA), NC (SNA), SC (SNA), TX (SNA) bBRNO Page 1 of 4 SUMMARY OF PREDICTED HABITAT BY MANAGMENT AND GAP PROTECTION STATUS: US FWS US Forest Service Tenn. Valley Author. US DOD/ACOE ha % ha % ha % ha % Status 1 0.0 0 0.0 0 0.0 0 0.0 0 Status 2 0.0 0 0.0 0 0.0 0 0.0 0 Status 3 0.0 0 0.0 0 0.0 0 0.0 0 Status 4 0.0 0 0.0 0 0.0 0 0.0 0 Total 0.0 0 0.0 0 0.0 0 0.0 0 US Dept. of Energy US Nat. Park Service NOAA Other Federal Lands ha % ha % ha % ha % Status 1 0.0 0 0.0 0 0.0 0 0.0 0 Status 2 0.0 0 0.0 0 0.0 0 0.0 0 Status 3 0.0 0 0.0 0 0.0 0 0.0 0 Status 4 0.0 0 0.0 0 0.0 0 0.0 0 Total 0.0 0 0.0 0 0.0 0 0.0 0 Native Am. -

Breeding Colonies Distribution of Brown Noddy Lineage
To view this as a map and many more go to: www.nabis.govt.nz web mapping tool Type the map name into: Search for a map layer or place Lineage – Scientific methodology Breeding distribution of Brown noddy lineage 1. A “breeding colony” for New Zealand seabirds is defined as “any location where breeding has been reported and is considered by the expert compiling the species account to have occurred at that location at least until 1998”. 2. An “occasional breeding colony” for New Zealand seabirds is defined as “any location where breeding has been reported, but not necessarily continuously nor during consecutive breeding seasons, and is considered by the expert compiling the species account to have occurred at that location during the last 30 years”. 3. Literature sources were searched for breeding distribution information. a. Scientific papers, published texts, unpublished reports and university theses available to the expert who prepared the distributional layers. b. Aquatic Sciences and Fisheries Abstracts for 1960-2009. c. OSNZ News and Southern Bird for 1977–2009. 4. Other sources. a. Nil. 5. The mapping of the Brown noddy breeding colony at Curtis Island is based on a written description of its location in Veitch et al. (2004) during 1989, those of the breeding colonies at Lord Howe Island and Norfolk Island are based on written descriptions of their locations in Higgins & Davies (1996), and that of the unknown breeding colony at L’Esperance Rock is based on a written description of its location in Greene et al. (2004) during 2002. The colonies have not been surveyed for mapping purposes, and the mapping presented is based on the written descriptions of their locations.