Breeding Colonies Distribution of Brown Noddy Lineage
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Conservation Problems on Tristan Da Cunha Byj
28 Oryx Conservation Problems on Tristan da Cunha ByJ. H. Flint The author spent two years, 1963-65, as schoolmaster on Tristan da Cunha, during which he spent four weeks on Nightingale Island. On the main island he found that bird stocks were being depleted and the islanders taking too many eggs and young; on Nightingale, however, where there are over two million pairs of great shearwaters, the harvest of these birds could be greater. Inaccessible Island, which like Nightingale, is without cats, dogs or rats, should be declared a wildlife sanctuary. Tl^HEN the first permanent settlers came to Tristan da Cunha in " the early years of the nineteenth century they found an island rich in bird and sea mammal life. "The mountains are covered with Albatross Mellahs Petrels Seahens, etc.," wrote Jonathan Lambert in 1811, and Midshipman Greene, who stayed on the island in 1816, recorded in his diary "Sea Elephants herding together in immense numbers." Today the picture is greatly changed. A century and a half of human habitation has drastically reduced the larger, edible species, and the accidental introduction of rats from a shipwreck in 1882 accelerated the birds' decline on the main island. Wood-cutting, grazing by domestic stock and, more recently, fumes from the volcano have destroyed much of the natural vegetation near the settlement, and two bird subspecies, a bunting and a flightless moorhen, have become extinct on the main island. Curiously, one is liable to see more birds on the day of arrival than in several weeks ashore. When I first saw Tristan from the decks of M.V. -

Red-Footed Booby Helper at Great Frigatebird Nests
264 SHORT COMMUNICATIONS NECTS MEANS ECTS MEANS ICATE SAMPLE SIZE S.D. SAMPLE SIZE 70 IN DAYS FIGURE 2. Culmen length against age of Brown FIGURE 1. Weight against age of Brown Noddy Noddy chicks on Manana Island, Hawaii in 1972. chicks on Manana Island, Hawaii in 1972. about the thirty-fifth day; apparently Brown Noddies on Christmas Island grow more rapidly than those on 5.26 g/day (SD = 1.18 g/day), and chick growth rate Manana. More data are required for a refined analysis and fledging age were negatively correlated (r = of intraspecific variation in growth rates of Brown -0.490, N = 19, P < 0.05). Noddy young. Seventeen of the chicks were weighed both at the This paper is based upon my doctoral dissertation age of fledging and from 3 to 12 days later; there was submitted to the University of Hawaii. I thank An- no significant recession in weight after fledging (t = drew J. Berger for guidance and criticism. The 1.17, P > 0.2), as suggested for certain terns (e.g., Hawaii State Division of Fish and Game kindly LeCroy and LeCroy 1974, Bird-Banding 45:326). granted me permission to work on Manana. This Dorward and Ashmole (1963, Ibis 103b: 447) mea- study was supported by the Department of Zoology sured growth in weight and culmen length of Brown of the University of Hawaii, by an NSF Graduate Noddies on Ascension Island in the Atlantic; scatter Fellowship, and by a Mount Holyoke College Faculty diagrams of their data indicate growth functions very Grant. -

Breeding Biology of the Brown Noddy on Tern Island, Hawaii
Wilson Bull., 108(2), 1996, pp. 317-334 BREEDING BIOLOGY OF THE BROWN NODDY ON TERN ISLAND, HAWAII JENNIFER L. MEGYESI’ AND CURTICE R. GRIFFINS ABSTRACT.-we observed Brown Noddy (Anous stolidus pileatus) breeding phenology and population trends on Tern Island, French Frigate Shoals, Hawaii, from 1982 to 1992. Peaks of laying ranged from the first week in January to the first week in November; however, most laying occurred between March and September each year. Incubation length was 34.8 days (N = 19, SD = 0.6, range = 29-37 days). There were no differences in breeding pairs between the measurements of the first egg laid and successive eggs laid within a season. The proportion of light- and dark-colored chicks was 26% and 74%, respectively (N = 221) and differed from other Brown Noddy colonies studied in Atlantic and Pacific oceans. The length of time between clutches depended on whether the previous outcome was a failed clutch or a successfully fledged chick. Hatching, fledging, and reproductive success were significantly different between years. The subspecies (A. s. pihtus) differs in many aspects of its breeding biology from other colonies in the Atlantic and Pacific oceans, in regard to year-round occurrence at the colony, frequent renesting attempts, large egg size, proportion of light and dark colored chicks, and low reproductive success caused by in- clement weather and predation by Great Frigatebirds (Fregata minor). Received 31 Mar., 1995, accepted 5 Dec. 1995. The Brown Noddy (Anous stolidus) is the largest and most widely distributed of the tropical and subtropical tern species (Cramp 1985). -
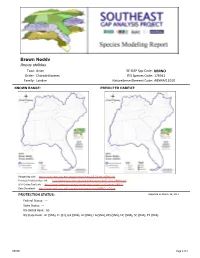
Brown Noddy Anous Stolidus Taxa: Avian SE-GAP Spp Code: Bbrno Order: Charadriiformes ITIS Species Code: 176941 Family: Laridae Natureserve Element Code: ABNNM11010
Brown Noddy Anous stolidus Taxa: Avian SE-GAP Spp Code: bBRNO Order: Charadriiformes ITIS Species Code: 176941 Family: Laridae NatureServe Element Code: ABNNM11010 KNOWN RANGE: PREDICTED HABITAT: P:\Proj1\SEGap P:\Proj1\SEGap Range Map Link: http://www.basic.ncsu.edu/segap/datazip/maps/SE_Range_bBRNO.pdf Predicted Habitat Map Link: http://www.basic.ncsu.edu/segap/datazip/maps/SE_Dist_bBRNO.pdf GAP Online Tool Link: http://www.gapserve.ncsu.edu/segap/segap/index2.php?species=bBRNO Data Download: http://www.basic.ncsu.edu/segap/datazip/region/vert/bBRNO_se00.zip PROTECTION STATUS: Reported on March 14, 2011 Federal Status: --- State Status: --- NS Global Rank: G5 NS State Rank: AL (SNA), FL (S1), GA (SNA), HI (SNR), LA (SNA), MS (SNA), NC (SNA), SC (SNA), TX (SNA) bBRNO Page 1 of 4 SUMMARY OF PREDICTED HABITAT BY MANAGMENT AND GAP PROTECTION STATUS: US FWS US Forest Service Tenn. Valley Author. US DOD/ACOE ha % ha % ha % ha % Status 1 0.0 0 0.0 0 0.0 0 0.0 0 Status 2 0.0 0 0.0 0 0.0 0 0.0 0 Status 3 0.0 0 0.0 0 0.0 0 0.0 0 Status 4 0.0 0 0.0 0 0.0 0 0.0 0 Total 0.0 0 0.0 0 0.0 0 0.0 0 US Dept. of Energy US Nat. Park Service NOAA Other Federal Lands ha % ha % ha % ha % Status 1 0.0 0 0.0 0 0.0 0 0.0 0 Status 2 0.0 0 0.0 0 0.0 0 0.0 0 Status 3 0.0 0 0.0 0 0.0 0 0.0 0 Status 4 0.0 0 0.0 0 0.0 0 0.0 0 Total 0.0 0 0.0 0 0.0 0 0.0 0 Native Am. -

Observations of Pelagic Seabirds Wintering at Sea in the Southeastern Caribbean William L
Pp. 104-110 in Studies in Trinidad and Tobago Ornithology Honouring Richard ffrench (F. E. Hayes and S. A. Temple, Eds.). Dept. Life Sci., Univ. West Indies, St. Augustine, Occ. Pap. 11, 2000 OBSERVATIONS OF PELAGIC SEABIRDS WINTERING AT SEA IN THE SOUTHEASTERN CARIBBEAN WILLIAM L. MURPHY, 8265 Glengarry Court, Indianapolis, IN 46236, USA ABSTRACT.-I report observations, including several the educational cruise ship Yorktown Clipper between significant distributional records, of 16 species of Curaçao and the Orinoco River, traversing seabirds wintering at sea in the southeastern Caribbean approximately 2,000 km per trip (Table 1). Because during cruises from Bonaire to the Orinoco River (5-13 the focus was on visiting islands as well as on cruising, January 1996, 3-12 March 1997, and 23 December many of the longer passages were traversed at night. 1997 - 1 January 1998). A few scattered shearwaters While at sea during the day, fellow birders and I (Calonectris diomedea and Puffinus lherminieri) were maintained a sea watch, recording sightings of bird seen. Storm-Petrels (Oceanites oceanicus and species and their numbers. Oceanodroma leucorhoa), particularly the latter species, were often seen toward the east. Most The observers were all experienced birders with tropicbirds (Phaethon aethereus) and gulls (Larus binoculars, some of which were image-stabilised. The atricilla) were near Tobago. Boobies were common; number of observers at any given time ranged from Sula leucogaster outnumbered S. sula by about 4:1 and one to 15, averaging about five. Observations were S. dactylatra was scarce. Frigatebirds (Fregata made from various points on three decks ranging from magnificens) were strictly coastal. -
![Metadata Also Available As - [Parseable Text] - [SGML] - [XML] Metadata](https://docslib.b-cdn.net/cover/4523/metadata-also-available-as-parseable-text-sgml-xml-metadata-2234523.webp)
Metadata Also Available As - [Parseable Text] - [SGML] - [XML] Metadata
Sensitivity of Coastal Environments and Wildlife to Spilled Oil: South Florida: BENTHIC (Benthic Polygons) Metadata also available as - [Parseable text] - [SGML] - [XML] Metadata: Identification_Information Data_Quality_Information Spatial_Data_Organization_Information Spatial_Reference_Information Entity_and_Attribute_Information Distribution_Information Metadata_Reference_Information Identification_Information: Citation: Citation_Information: Originator: National Oceanic and Atmospheric Administration (NOAA), National Ocean Service (NOS), Office of Response and Restoration (OR&R), Emergency Response Division (ERD), Seattle, Washington. Publication_Date: 201304 Title: Sensitivity of Coastal Environments and Wildlife to Spilled Oil: South Florida: BENTHIC (Benthic Polygons) Edition: Second Geospatial_Data_Presentation_Form: vector digital data Series_Information: Series_Name: South Florida Issue_Identification: South Florida Publication_Information: Publication_Place: Seattle, Washington Publisher: NOAA's Ocean Service, Office of Response and Restoration (OR&R), Emergency Response Division (ERD). Other_Citation_Details: Prepared by Research Planning, Inc., Columbia, South Carolina for the National Oceanic and Atmospheric Administration (NOAA), National Ocean Service, Office of Response and Restoration, Emergency Response Division, Seattle, Washington. Online_Linkage: <http://response.restoration.noaa.gov/esi> Description: Abstract: This data set contains benthic habitats, including: coral reef and hardbottom, seagrass, algae, and others -

Anous Stolidus (Brown Noddy Or Common Noddy)
UWI The Online Guide to the Animals of Trinidad and Tobago Ecology Anous stolidus (Brown Noddy or Common Noddy) Family: Laridae (Gulls and Terns) Order: Charadriiformes (Shorebirds and Waders) Class: Aves (Birds) Fig. 1. Brown noddy, Anous stolidus. [http://www.oiseaux-birds.com/card-brown-noddy.html, downloaded 16 February 2017] TRAITS. The brown noddy is recognized from other terns (sea birds) as a dark coloured bird with a distinct white colour on its head and a straight tail (Fig. 1), compared to other terns that are white with a black cap and a forked tail (Ffrench, 1991). The brown noddy has an average size of approximately 38cm at adulthood (Ffrench, 2012). This species is sexually dimorphic as the males and females are different; the male is considerably larger than the female in all aspects of the body (Chardine and Morris, 1989). DISTRIBUTION. The brown noddy is a tropical bird that has a geographic range throughout the regions of the world (Fig. 2). It can be found in many including the Caribbean Sea (IUCN, 2017). HABITAT AND ACTIVITY. Anous stolidus is found on coastal and oceanic islands during the breeding season (Bouglouan, 2017) or along coasts. When foraging, they fly close to the sea in order to catch their prey (Audubon, 2017). The noddy can be found on coastal rocks located on the steep side of a cliff where they normally form nests. The nests generally consist of pieces of sticks strung together with seaweed and are commonly lined with rocks and coral from the shore UWI The Online Guide to the Animals of Trinidad and Tobago Ecology (Ffrench, 2012). -

Threats to Seabirds: a Global Assessment 2 3 4 Authors: Maria P
1 Threats to seabirds: a global assessment 2 3 4 Authors: Maria P. Dias1*, Rob Martin1, Elizabeth J. Pearmain1, Ian J. Burfield1, Cleo Small2, Richard A. 5 Phillips3, Oliver Yates4, Ben Lascelles1, Pablo Garcia Borboroglu5, John P. Croxall1 6 7 8 Affiliations: 9 1 - BirdLife International. The David Attenborough Building, Pembroke Street Cambridge CB2 3QZ UK 10 2 - BirdLife International Marine Programme, RSPB, The Lodge, Sandy, SG19 2DL 11 3 – British Antarctic Survey. Natural Environment Research Council, High Cross, Madingley Road, 12 Cambridge CB3 0ET, UK 13 4 – Centre for the Environment, Fishery and Aquaculture Science, Pakefield Road, Lowestoft, NR33, UK 14 5 - Global Penguin Society, University of Washington and CONICET Argentina. Puerto Madryn U9120, 15 Chubut, Argentina 16 * Corresponding author: Maria Dias, [email protected]. BirdLife International. The David 17 Attenborough Building, Pembroke Street Cambridge CB2 3QZ UK. Phone: +44 (0)1223 747540 18 19 20 Acknowledgements 21 We are very grateful to Bartek Arendarczyk, Sophie Bennett, Ricky Hibble, Eleanor Miller and Amy 22 Palmer-Newton for assisting with the bibliographic review. We thank Rachael Alderman, Pep Arcos, 23 Jonathon Barrington, Igor Debski, Peter Hodum, Gustavo Jimenez, Jeff Mangel, Ken Morgan, Paul Sagar, 24 Peter Ryan, and other members of the ACAP PaCSWG, and the members of IUCN SSC Penguin Specialist 25 Group (Alejandro Simeone, Andre Chiaradia, Barbara Wienecke, Charles-André Bost, Lauren Waller, Phil 26 Trathan, Philip Seddon, Susie Ellis, Tom Schneider and Dee Boersma) for reviewing threats to selected 27 species. We thank also Andy Symes, Rocio Moreno, Stuart Butchart, Paul Donald, Rory Crawford, 28 Tammy Davies, Ana Carneiro and Tris Allinson for fruitful discussions and helpful comments on earlier 29 versions of the manuscript. -

Coral Cap Species of Flower Garden Banks National Marine Sanctuary
CORAL CAP SPECIES OF FLOWER GARDEN BANKS NATIONAL MARINE SANCTUARY Classification Common name Scientific Name Bacteria Schizothrix calcicola CORAL CAP SPECIES OF FLOWER GARDEN BANKS NATIONAL MARINE SANCTUARY Classification Common name Scientific Name Algae Brown Algae Dictyopteris justii Forded Sea Tumbleweeds Dictyota bartayresii Dictyota cervicornis Dictyota dichotoma Dictyota friabilis (pfaffii) Dictyota humifusa Dictyota menstrualis Dictyota pulchella Ectocarpus elachistaeformis Leathery Lobeweeds, Encrusting Lobophora variegata Fan-leaf Alga Peacock's Tail Padina jamaicensis Padina profunda Padina sanctae-crucis Rosenvingea intricata Gulf Weed, Sargassum Weed Sargassum fluitans White-vein Sargassum Sargassum hystrix Sargasso Weed Sargassum natans Spatoglossum schroederi Sphacelaria tribuloides Sphacelaria Rigidula Leafy Flat-blade Alga Stypopodium zonale Green Algae Papyrus Print Alga Anadyomene stellata Boodelopsis pusilla Bryopsis plumosa Bryopsis pennata Caulerpa microphysa Caulerpa peltata Green Grape Alga Caulerpa racemosa v. macrophysa Cladophora cf. repens Cladophoropsis membranacea Codium decorticatum Dead Man’s Fingers Codium isthmocladum Codium taylori Hair Algae Derbesia cf. marina Entocladia viridis Large Leaf Watercress Alga Halimeda discoidea Halimeda gracilis Green Net Alga Microdictyon boergesenii Spindleweed, Fuzzy Tip Alga Neomeris annulata Struvea sp. CORAL CAP SPECIES OF FLOWER GARDEN BANKS NATIONAL MARINE SANCTUARY Classification Common name Scientific Name Udotea flabellum Ulva lactuca Ulvella lens Elongated -

Order CHARADRIIFORMES: Waders, Gulls and Terns Suborder LARI
Text extracted from Gill B.J.; Bell, B.D.; Chambers, G.K.; Medway, D.G.; Palma, R.L.; Scofield, R.P.; Tennyson, A.J.D.; Worthy, T.H. 2010. Checklist of the birds of New Zealand, Norfolk and Macquarie Islands, and the Ross Dependency, Antarctica. 4th edition. Wellington, Te Papa Press and Ornithological Society of New Zealand. Pages 191, 223 & 230-231. Order CHARADRIIFORMES: Waders, Gulls and Terns The family sequence of Christidis & Boles (1994), who adopted that of Sibley et al. (1988) and Sibley & Monroe (1990), is followed here. Suborder LARI: Skuas, Gulls, Terns and Skimmers Condon (1975) and Checklist Committee (1990) recognised three subfamilies within the Laridae (Larinae, Sterninae and Megalopterinae) but this division has not been widely adopted. We follow Gochfeld & Burger (1996) in recognising gulls in one family (Laridae) and terns and noddies in another (Sternidae). The sequence of species for Stercorariidae and Laridae follows Peters (1934) and for Sternidae follows Bridge et al. (2005). Family STERNIDAE Bonaparte: Terns and Noddies Sterninae Bonaparte, 1838: Geogr. Comp. List. Birds: 61 – Type genus Sterna Linnaeus, 1758. Most recommendations from a new study of tern and noddy relationships, based on mtDNA (Bridge et al. 2005), have already been adopted by the Taxonomic Subcommittee of the British Ornithologists’ Union Records Committee (Sangster et al. 2005) and the American Ornithologists’ Union Committee on Classification and Nomenclature (Banks, R.C. et al. 2006). This follows many years of disagreement about the generic classification of terns for which 3–12 genera have recently been used (see Bridge et al. 2005). The genera and their sequence recommended by Bridge et al. -

Noio Kōhā Or Brown Noddy Anous Stolidus
Seabirds Noio Kōhā or Brown Noddy Anous stolidus SPECIES STATUS: State recognized as Indigenous NatureServe Heritage Rank G5 - Secure Photo: Forest and Kim Starr, USFWS North American Waterbird Conservation Plan – Not currently at risk Regional Seabird Conservation Plan - USFWS 2005 SPECIES INFORMATION: The noio kōhā or brown noddy is a medium-sized, abundant tern (Family: Laridae) with a pantropical distribution, and is very similar to noio (black noddy) in appearance and behavior. Five subspecies of noio kōhā (brown noddy) are recognized, and one (A. s. pileatus) is resident in Hawai‘i. Individuals have slender wings and a wedge-shaped tail. Adult males and females are dark brown with a white cap and have a black bill, legs, and feet; males are larger than females. Flight is swift with rapid wing beats and usually direct and low over the ocean, this species almost never soars high. Often forages in large, mixed species flocks associated with schools of large predatory fishes which drive prey species to the surface. Noio kōhā (brown noddy) generally forage in nearshore waters and mainly feed by dipping the surface from the wing or by making shallow dives. In Hawai‘i, diet is comprised mostly of fish, but squid are also taken. Breed in large, dense colonies and nest on the ground, on cliffs or in trees. In Hawai‘i, breeding is synchronous with peaks occurring in the spring and summer. Pairs stay together throughout the year, but there is little information on the length of pair bonds. Both parents incubate the single egg, and brood and feed chick. -

Arabian Peninsula
THE CONSERVATION STATUS AND DISTRIBUTION OF THE BREEDING BIRDS OF THE ARABIAN PENINSULA Compiled by Andy Symes, Joe Taylor, David Mallon, Richard Porter, Chenay Simms and Kevin Budd ARABIAN PENINSULA The IUCN Red List of Threatened SpeciesTM - Regional Assessment About IUCN IUCN, International Union for Conservation of Nature, helps the world find pragmatic solutions to our most pressing environment and development challenges. IUCN’s work focuses on valuing and conserving nature, ensuring effective and equitable governance of its use, and deploying nature-based solutions to global challenges in climate, food and development. IUCN supports scientific research, manages field projects all over the world, and brings governments, NGOs, the UN and companies together to develop policy, laws and best practice. IUCN is the world’s oldest and largest global environmental organization, with almost 1,300 government and NGO Members and more than 15,000 volunteer experts in 185 countries. IUCN’s work is supported by almost 1,000 staff in 45 offices and hundreds of partners in public, NGO and private sectors around the world. www.iucn.org About the Species Survival Commission The Species Survival Commission (SSC) is the largest of IUCN’s six volunteer commissions with a global membership of around 7,500 experts. SSC advises IUCN and its members on the wide range of technical and scientific aspects of species conservation, and is dedicated to securing a future for biodiversity. SSC has significant input into the international agreements dealing with biodiversity conservation. About BirdLife International BirdLife International is the world’s largest nature conservation Partnership. BirdLife is widely recognised as the world leader in bird conservation.