Lord Howe Island Rodent Eradication Project DRAFT Public Environment Report EPBC 2016/7703
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Conservation Problems on Tristan Da Cunha Byj
28 Oryx Conservation Problems on Tristan da Cunha ByJ. H. Flint The author spent two years, 1963-65, as schoolmaster on Tristan da Cunha, during which he spent four weeks on Nightingale Island. On the main island he found that bird stocks were being depleted and the islanders taking too many eggs and young; on Nightingale, however, where there are over two million pairs of great shearwaters, the harvest of these birds could be greater. Inaccessible Island, which like Nightingale, is without cats, dogs or rats, should be declared a wildlife sanctuary. Tl^HEN the first permanent settlers came to Tristan da Cunha in " the early years of the nineteenth century they found an island rich in bird and sea mammal life. "The mountains are covered with Albatross Mellahs Petrels Seahens, etc.," wrote Jonathan Lambert in 1811, and Midshipman Greene, who stayed on the island in 1816, recorded in his diary "Sea Elephants herding together in immense numbers." Today the picture is greatly changed. A century and a half of human habitation has drastically reduced the larger, edible species, and the accidental introduction of rats from a shipwreck in 1882 accelerated the birds' decline on the main island. Wood-cutting, grazing by domestic stock and, more recently, fumes from the volcano have destroyed much of the natural vegetation near the settlement, and two bird subspecies, a bunting and a flightless moorhen, have become extinct on the main island. Curiously, one is liable to see more birds on the day of arrival than in several weeks ashore. When I first saw Tristan from the decks of M.V. -

Red-Footed Booby Helper at Great Frigatebird Nests
264 SHORT COMMUNICATIONS NECTS MEANS ECTS MEANS ICATE SAMPLE SIZE S.D. SAMPLE SIZE 70 IN DAYS FIGURE 2. Culmen length against age of Brown FIGURE 1. Weight against age of Brown Noddy Noddy chicks on Manana Island, Hawaii in 1972. chicks on Manana Island, Hawaii in 1972. about the thirty-fifth day; apparently Brown Noddies on Christmas Island grow more rapidly than those on 5.26 g/day (SD = 1.18 g/day), and chick growth rate Manana. More data are required for a refined analysis and fledging age were negatively correlated (r = of intraspecific variation in growth rates of Brown -0.490, N = 19, P < 0.05). Noddy young. Seventeen of the chicks were weighed both at the This paper is based upon my doctoral dissertation age of fledging and from 3 to 12 days later; there was submitted to the University of Hawaii. I thank An- no significant recession in weight after fledging (t = drew J. Berger for guidance and criticism. The 1.17, P > 0.2), as suggested for certain terns (e.g., Hawaii State Division of Fish and Game kindly LeCroy and LeCroy 1974, Bird-Banding 45:326). granted me permission to work on Manana. This Dorward and Ashmole (1963, Ibis 103b: 447) mea- study was supported by the Department of Zoology sured growth in weight and culmen length of Brown of the University of Hawaii, by an NSF Graduate Noddies on Ascension Island in the Atlantic; scatter Fellowship, and by a Mount Holyoke College Faculty diagrams of their data indicate growth functions very Grant. -

A Classification of the Rallidae
A CLASSIFICATION OF THE RALLIDAE STARRY L. OLSON HE family Rallidae, containing over 150 living or recently extinct species T and having one of the widest distributions of any family of terrestrial vertebrates, has, in proportion to its size and interest, received less study than perhaps any other major group of birds. The only two attempts at a classifi- cation of all of the recent rallid genera are those of Sharpe (1894) and Peters (1934). Although each of these lists has some merit, neither is satisfactory in reflecting relationships between the genera and both often separate closely related groups. In the past, no attempt has been made to identify the more primitive members of the Rallidae or to illuminate evolutionary trends in the family. Lists almost invariably begin with the genus Rdus which is actually one of the most specialized genera of the family and does not represent an ancestral or primitive stock. One of the difficulties of rallid taxonomy arises from the relative homo- geneity of the family, rails for the most part being rather generalized birds with few groups having morphological modifications that clearly define them. As a consequence, particularly well-marked genera have been elevated to subfamily rank on the basis of characters that in more diverse families would not be considered as significant. Another weakness of former classifications of the family arose from what Mayr (194933) referred to as the “instability of the morphology of rails.” This “instability of morphology,” while seeming to belie what I have just said about homogeneity, refers only to the characteristics associated with flightlessness-a condition that appears with great regularity in island rails and which has evolved many times. -

New Zealand Fishes a Field Guide to Common Species Caught by Bottom, Midwater, and Surface Fishing Cover Photos: Top – Kingfish (Seriola Lalandi), Malcolm Francis
New Zealand fishes A field guide to common species caught by bottom, midwater, and surface fishing Cover photos: Top – Kingfish (Seriola lalandi), Malcolm Francis. Top left – Snapper (Chrysophrys auratus), Malcolm Francis. Centre – Catch of hoki (Macruronus novaezelandiae), Neil Bagley (NIWA). Bottom left – Jack mackerel (Trachurus sp.), Malcolm Francis. Bottom – Orange roughy (Hoplostethus atlanticus), NIWA. New Zealand fishes A field guide to common species caught by bottom, midwater, and surface fishing New Zealand Aquatic Environment and Biodiversity Report No: 208 Prepared for Fisheries New Zealand by P. J. McMillan M. P. Francis G. D. James L. J. Paul P. Marriott E. J. Mackay B. A. Wood D. W. Stevens L. H. Griggs S. J. Baird C. D. Roberts‡ A. L. Stewart‡ C. D. Struthers‡ J. E. Robbins NIWA, Private Bag 14901, Wellington 6241 ‡ Museum of New Zealand Te Papa Tongarewa, PO Box 467, Wellington, 6011Wellington ISSN 1176-9440 (print) ISSN 1179-6480 (online) ISBN 978-1-98-859425-5 (print) ISBN 978-1-98-859426-2 (online) 2019 Disclaimer While every effort was made to ensure the information in this publication is accurate, Fisheries New Zealand does not accept any responsibility or liability for error of fact, omission, interpretation or opinion that may be present, nor for the consequences of any decisions based on this information. Requests for further copies should be directed to: Publications Logistics Officer Ministry for Primary Industries PO Box 2526 WELLINGTON 6140 Email: [email protected] Telephone: 0800 00 83 33 Facsimile: 04-894 0300 This publication is also available on the Ministry for Primary Industries website at http://www.mpi.govt.nz/news-and-resources/publications/ A higher resolution (larger) PDF of this guide is also available by application to: [email protected] Citation: McMillan, P.J.; Francis, M.P.; James, G.D.; Paul, L.J.; Marriott, P.; Mackay, E.; Wood, B.A.; Stevens, D.W.; Griggs, L.H.; Baird, S.J.; Roberts, C.D.; Stewart, A.L.; Struthers, C.D.; Robbins, J.E. -

And Long-Term Movement Patterns of Six Temperate Reef Fishes (Families Labridae and Monacanthidae)
Mar: Freshwater Res., 1995,46, 853-60 Short- and Long-term Movement Patterns of Six Temperate Reef Fishes (Families Labridae and Monacanthidae) Neville S. Barrett Zoology Department, University of Tasmania, GPO Box 252C Hobart, Tas. 7001, Australia. Abstract. Movement patterns were studied on a 1-ha isolated reef surrounding Arch Rock in southern Tasmania. Short-term movements were identified from diver observations, and interpretation of long-term movements involved multiple recaptures of tagged individuals. Visual observations indicated that the sex-changing labrids Notolabrus tetricus, Pictilabrus laticlavius and Pseudolabrus psittaculus were all site-attached, with females having overlapping home ranges and males being territorial. In the non-sex-changing labrid Notolabrus fucicola and in the monacanthids Penicipelta vittiger and Meuschenia australis, there was no evidence of territorial behaviour and I-h movements were in excess of the scale of the study. The long-term results indicated that all species were permanent reef residents, with most individuals of all species except M. australis always being recaptured within a home range of 100 m X 25 m or less. Only 15% of individuals of M. australis were always recaptured within this range category. The natural habitat boundary of open sand between the Arch Rock reef and adjacent reefs appeared to be an effective deterrent to emigration. The use of natural boundaries should be an important consideration in the design of marine reserves where the aim is to minimize the loss of protected species to adjacent fished areas. Introduction associated with using SCUBA in colder waters with low Most tropical reef fishes are now regarded as sedentary, visibility. -

Breeding Biology of the Brown Noddy on Tern Island, Hawaii
Wilson Bull., 108(2), 1996, pp. 317-334 BREEDING BIOLOGY OF THE BROWN NODDY ON TERN ISLAND, HAWAII JENNIFER L. MEGYESI’ AND CURTICE R. GRIFFINS ABSTRACT.-we observed Brown Noddy (Anous stolidus pileatus) breeding phenology and population trends on Tern Island, French Frigate Shoals, Hawaii, from 1982 to 1992. Peaks of laying ranged from the first week in January to the first week in November; however, most laying occurred between March and September each year. Incubation length was 34.8 days (N = 19, SD = 0.6, range = 29-37 days). There were no differences in breeding pairs between the measurements of the first egg laid and successive eggs laid within a season. The proportion of light- and dark-colored chicks was 26% and 74%, respectively (N = 221) and differed from other Brown Noddy colonies studied in Atlantic and Pacific oceans. The length of time between clutches depended on whether the previous outcome was a failed clutch or a successfully fledged chick. Hatching, fledging, and reproductive success were significantly different between years. The subspecies (A. s. pihtus) differs in many aspects of its breeding biology from other colonies in the Atlantic and Pacific oceans, in regard to year-round occurrence at the colony, frequent renesting attempts, large egg size, proportion of light and dark colored chicks, and low reproductive success caused by in- clement weather and predation by Great Frigatebirds (Fregata minor). Received 31 Mar., 1995, accepted 5 Dec. 1995. The Brown Noddy (Anous stolidus) is the largest and most widely distributed of the tropical and subtropical tern species (Cramp 1985). -

Impact of Sea Level Rise on Coastal Natural Values in Tasmania
Impact of sea level rise on coastal natural values in Tasmania JUNE 2016 Department of Primary Industries, Parks, Water and Environment Acknowledgements Thanks to the support we received in particular from Clarissa Murphy who gave six months as a volunteer in the first phase of the sea level rise risk assessment work. We also had considerable technical input from a range of people on various aspects of the work, including Hans and Annie Wapstra, Richard Schahinger, Tim Rudman, John Church, and Anni McCuaig. We acknowledge the hard work over a number of years from the Sea Level Rise Impacts Working Group: Oberon Carter, Louise Gilfedder, Felicity Faulkner, Lynne Sparrow (DPIPWE), Eric Woehler (BirdLife Tasmania) and Chris Sharples (University of Tasmania). This report was compiled by Oberon Carter, Felicity Faulkner, Louise Gilfedder and Peter Voller from the Natural Values Conservation Branch. Citation DPIPWE (2016) Impact of sea level rise on coastal natural values in Tasmania. Natural and Cultural Heritage Division, Department of Primary Industries, Parks, Water and Environment, Hobart. www.dpipwe.tas.gov.au ISBN: 978-1-74380-009-6 Cover View to Mount Cameron West by Oberon Carter. Pied Oystercatcher by Mick Brown. The Pied Oystercatcher is considered to have a very high exposure to sea level rise under both a national assessment and Tasmanian assessment. Its preferred habitat is mudflats, sandbanks and sandy ocean beaches, all vulnerable to inundation and erosion. Round-leaved Pigface (Disphyma australe) in flower in saltmarsh at Lauderdale by Iona Mitchell. Three saltmarsh communities are associated with the coastal zone and are considered at risk from sea level rise. -

Rail (Hypotaenidia Okinawae)
Community Engagement with Wildlife Conservation in Japan: A Case Study of an Endangered Bird, the Okinawa Rail (Hypotaenidia okinawae) MADELEINE SBEGHEN University of Queensland ABSTRACT As host of the 2010 Nagoya Biodiversity Summit, Japan reaffi rmed its eff orts to conserve biodiversity for future generations. Rebuilding relationships with nature and strengthening conservation education are key priorities of Japan’s biodiversity conservation agenda to improve outcomes for threatened species and local communities. Th is paper examines community engagement with the critically endangered Okinawa Rail (Hypotaenidia okinawae), an endemic bird of the Yanbaru forests of northern Okinawa, with reference to the conservation context in Japan. Since discovery of the Okinawa Rail in 1981, communities in Yanbaru have developed a strong relationship with this species, recognising it as an important symbol of regional cultural identity and as a unique ecological asset that attracts visitors and underpins community events. Th is has translated into investment by government and community stakeholders in conservation education facilities and public awareness campaigns for To link to this article: the Okinawa Rail in Yanbaru. To improve the long-term value of facilities http://doi.org/10.21159/nvjs.09.01 to support science-based conservation eff orts in this Japanese context, it ISSN 2205-3166 could be advantageous to increase opportunities for social learning that New Voices in Japanese Studies is incorporate both educational and conservation goals, and which encourage an interdisciplinary, peer-reviewed journal showcasing the work of stakeholder partnerships. Th e complex socio-economic and political context emerging scholars from Australia in Okinawa, and the signifi cant impact human activities have on the Okinawa and New Zealand with research interests in Japan. -

Discover the History of Warrnambool's Streets
Discover the history of Warrnambool's streets Street Name Description Locality Length Origin of Street Name Abbey Lane A laneway running between Hyland and Hart Streets, south of Timor Warrnambool 495m Benjamin Abbey (1862-1943) served two terms as Councillor 1913-16 and 1920-30. Served as Mayor 1924-26 during the Street. building of the Municipal Chambers. He was Manager of the Warrnambool branch of the Co-Operative Box Works of Victoria situated in South Warrnambool and a Trustee of the Methodist Church. His first wife Annie (nee Newman) died in Appears, unnamed, on an 1890 map. 1916 and his 2nd wife, Anastasia, died in 1994. This unnamed road was named Abbey Lane by the City of Warrnambool on 29th April 1991. The Council minutes and Government Gazette specifically name only the section between Hart and Hyland Streets which means the section between Hart and Ryot Streets is technically still unnamed. Aberline Road A northerly continuation of McKiernan Road, running from the Moore Warrnambool 1917m Joseph Aberline (1809-1874) arrived in Warrnambool in 1849 after spending some years in New Zealand. His property, Street/Dales Road intersection north to Wangoom Road. "The Grove", built on Wangoom Road in the 1860s was the site of a brick-making enterprise established by his son, John (1854-1940) in 1891. It was from the Wangoom Road property that large boulders were taken for use as some of the filling A very old road that appears on an 1856 map of Warrnambool. for the Warrnambool breakwater. Some older maps call it Aberlines Road. -
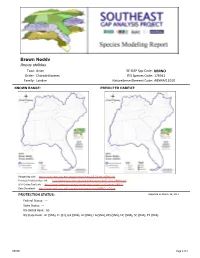
Brown Noddy Anous Stolidus Taxa: Avian SE-GAP Spp Code: Bbrno Order: Charadriiformes ITIS Species Code: 176941 Family: Laridae Natureserve Element Code: ABNNM11010
Brown Noddy Anous stolidus Taxa: Avian SE-GAP Spp Code: bBRNO Order: Charadriiformes ITIS Species Code: 176941 Family: Laridae NatureServe Element Code: ABNNM11010 KNOWN RANGE: PREDICTED HABITAT: P:\Proj1\SEGap P:\Proj1\SEGap Range Map Link: http://www.basic.ncsu.edu/segap/datazip/maps/SE_Range_bBRNO.pdf Predicted Habitat Map Link: http://www.basic.ncsu.edu/segap/datazip/maps/SE_Dist_bBRNO.pdf GAP Online Tool Link: http://www.gapserve.ncsu.edu/segap/segap/index2.php?species=bBRNO Data Download: http://www.basic.ncsu.edu/segap/datazip/region/vert/bBRNO_se00.zip PROTECTION STATUS: Reported on March 14, 2011 Federal Status: --- State Status: --- NS Global Rank: G5 NS State Rank: AL (SNA), FL (S1), GA (SNA), HI (SNR), LA (SNA), MS (SNA), NC (SNA), SC (SNA), TX (SNA) bBRNO Page 1 of 4 SUMMARY OF PREDICTED HABITAT BY MANAGMENT AND GAP PROTECTION STATUS: US FWS US Forest Service Tenn. Valley Author. US DOD/ACOE ha % ha % ha % ha % Status 1 0.0 0 0.0 0 0.0 0 0.0 0 Status 2 0.0 0 0.0 0 0.0 0 0.0 0 Status 3 0.0 0 0.0 0 0.0 0 0.0 0 Status 4 0.0 0 0.0 0 0.0 0 0.0 0 Total 0.0 0 0.0 0 0.0 0 0.0 0 US Dept. of Energy US Nat. Park Service NOAA Other Federal Lands ha % ha % ha % ha % Status 1 0.0 0 0.0 0 0.0 0 0.0 0 Status 2 0.0 0 0.0 0 0.0 0 0.0 0 Status 3 0.0 0 0.0 0 0.0 0 0.0 0 Status 4 0.0 0 0.0 0 0.0 0 0.0 0 Total 0.0 0 0.0 0 0.0 0 0.0 0 Native Am. -

Ornithol. Sci. 18(2): 169-175
Ornithol Sci 18: 169 – 175 (2019) REVIEW ARTICLE Preventing the extinction of the Lord Howe Woodhen (Hypotaenidia sylvestris) through predator eradication and population augmentation Dean PORTELLI1 and Nicholas CARLILE2,# 1 Department of Environment and Natural Resources, P.O. Box 1120, Alice Springs, NT, 0871 Australia 2 Office of Environment and Heritage, P.O. Box 1967, Hurstville, NSW, 2220 Australia ORNITHOLOGICAL Abstract The Lord Howe Woodhen (Hypotaenidia sylvestris) is endemic to Lord Howe Island off the mid-east coast of Australia and came perilously close to extinc- SCIENCE tion as a result of hunting pressure and introduced predators. A recovery program was © The Ornithological Society implemented in the 1970s to reverse the decline of the species through eradicating of Japan 2019 introduced predators (pigs, cats and goats) and augmenting the population through an in situ captive-breeding program. In 1980, three wild breeding pairs were taken into captivity from Mount Gower. Over the four years of the captive-breeding program, 76 chicks were produced from the original founders and their progeny and an additional four chicks were artificially reared from eggs collected from a wild pair. Almost all woodhens were liberated across four release sites, but only 13% of released birds were resighted and numbers increased at only one of these sites and then declined. A captive-bred female that was released into the lowlands paired with a wild male (which had been temporarily held in captivity) and bred prolifically, leading to rapid population growth in the lowlands. The subpopulation on Mount Gower increased fivefold in the decade following the captive-breeding program, despite the removal of the three breeding pairs (which were released elsewhere) and receiving no aug- mentation from the captive-breeding program. -

Breeding Colonies Distribution of Brown Noddy Lineage
To view this as a map and many more go to: www.nabis.govt.nz web mapping tool Type the map name into: Search for a map layer or place Lineage – Scientific methodology Breeding distribution of Brown noddy lineage 1. A “breeding colony” for New Zealand seabirds is defined as “any location where breeding has been reported and is considered by the expert compiling the species account to have occurred at that location at least until 1998”. 2. An “occasional breeding colony” for New Zealand seabirds is defined as “any location where breeding has been reported, but not necessarily continuously nor during consecutive breeding seasons, and is considered by the expert compiling the species account to have occurred at that location during the last 30 years”. 3. Literature sources were searched for breeding distribution information. a. Scientific papers, published texts, unpublished reports and university theses available to the expert who prepared the distributional layers. b. Aquatic Sciences and Fisheries Abstracts for 1960-2009. c. OSNZ News and Southern Bird for 1977–2009. 4. Other sources. a. Nil. 5. The mapping of the Brown noddy breeding colony at Curtis Island is based on a written description of its location in Veitch et al. (2004) during 1989, those of the breeding colonies at Lord Howe Island and Norfolk Island are based on written descriptions of their locations in Higgins & Davies (1996), and that of the unknown breeding colony at L’Esperance Rock is based on a written description of its location in Greene et al. (2004) during 2002. The colonies have not been surveyed for mapping purposes, and the mapping presented is based on the written descriptions of their locations.