Some Basic Chemistry
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Pre-Activity Quiz Answer Key (Pdf)
Name: ____________________________________________ Date: ___________________ Class: ________________________ Pre-Activity Quiz Answer Key 1. Consider a molecule of carbon monoxide (C O): A. Do you think the electrons in the triple bond pull closer to the C atom or the O atom, or are they equally shared? Use the concept of electronegativity to explain your response. Answer: They are closer to the O atom. Using the electronegativity scale from a textbook, the result is a polar covalent bond shifted towards the O atom. B. Is the bond polar or non-polar? Polar 2. In today’s engineering challenge, you will sketch out Lewis dot diagrams for various molecules and polyatomic ions. Then you will construct each molecule using a molecular model kit. The kits contain three different representations: colored balls, short sticks and long flexible springs. A. Each colored ball corresponds to a different atom. How can you determine which color to use for each atom? Use the reference sheet that comes with the kit. B. For what bond type do you think the short sticks are used? Covalent bonds C. If you were to build a triple bond, what would you use to represent a triple bond and how many would you use? Use springs, three springs 3. You will become familiar with different geometries of simple molecules. A. Name the theory used to predict molecular shapes of these molecules? VSEPR theory Molecular Models and 3-D Printing Activity—Pre-Activity Quiz Answer Key 1 Name: ____________________________________________ Date: ___________________ Class: ________________________ B. What if a molecule contains a central atom bonded to two identical outer atoms with the central atom surrounded by a lone pair of electrons? Name the geometry of this molecule. -

Neutron Stars
Chandra X-Ray Observatory X-Ray Astronomy Field Guide Neutron Stars Ordinary matter, or the stuff we and everything around us is made of, consists largely of empty space. Even a rock is mostly empty space. This is because matter is made of atoms. An atom is a cloud of electrons orbiting around a nucleus composed of protons and neutrons. The nucleus contains more than 99.9 percent of the mass of an atom, yet it has a diameter of only 1/100,000 that of the electron cloud. The electrons themselves take up little space, but the pattern of their orbit defines the size of the atom, which is therefore 99.9999999999999% Chandra Image of Vela Pulsar open space! (NASA/PSU/G.Pavlov et al. What we perceive as painfully solid when we bump against a rock is really a hurly-burly of electrons moving through empty space so fast that we can't see—or feel—the emptiness. What would matter look like if it weren't empty, if we could crush the electron cloud down to the size of the nucleus? Suppose we could generate a force strong enough to crush all the emptiness out of a rock roughly the size of a football stadium. The rock would be squeezed down to the size of a grain of sand and would still weigh 4 million tons! Such extreme forces occur in nature when the central part of a massive star collapses to form a neutron star. The atoms are crushed completely, and the electrons are jammed inside the protons to form a star composed almost entirely of neutrons. -
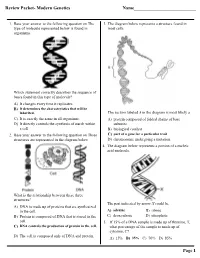
Review Packet- Modern Genetics Name___Page 1
Review Packet- Modern Genetics Name___________________________ 1. Base your answer to the following question on The 3. The diagram below represents a structure found in type of molecule represented below is found in most cells. organisms. Which statement correctly describes the sequence of bases found in this type of molecule? A) It changes every time it replicates. B) It determines the characteristics that will be inherited. The section labeled A in the diagram is most likely a C) It is exactly the same in all organisms. A) protein composed of folded chains of base D) It directly controls the synthesis of starch within subunits a cell. B) biological catalyst 2. Base your answer to the following question on Three C) part of a gene for a particular trait structures are represented in the diagram below. D) chromosome undergoing a mutation 4. The diagram below represents a portion of a nucleic acid molecule. What is the relationship between these three structures? The part indicated by arrow X could be A) DNA is made up of proteins that are synthesized in the cell. A) adenine B) ribose B) Protein is composed of DNA that is stored in the C) deoxyribose D) phosphate cell. 5. If 15% of a DNA sample is made up of thymine, T, C) DNA controls the production of protein in the cell. what percentage of the sample is made up of cytosine, C? D) The cell is composed only of DNA and protein. A) 15% B) 35% C) 70% D) 85% Page 1 6. Base your answer to the following question on Which 10.The diagram below represents genetic material. -

An Alternate Graphical Representation of Periodic Table of Chemical Elements Mohd Abubakr1, Microsoft India (R&D) Pvt
An Alternate Graphical Representation of Periodic table of Chemical Elements Mohd Abubakr1, Microsoft India (R&D) Pvt. Ltd, Hyderabad, India. [email protected] Abstract Periodic table of chemical elements symbolizes an elegant graphical representation of symmetry at atomic level and provides an overview on arrangement of electrons. It started merely as tabular representation of chemical elements, later got strengthened with quantum mechanical description of atomic structure and recent studies have revealed that periodic table can be formulated using SO(4,2) SU(2) group. IUPAC, the governing body in Chemistry, doesn‟t approve any periodic table as a standard periodic table. The only specific recommendation provided by IUPAC is that the periodic table should follow the 1 to 18 group numbering. In this technical paper, we describe a new graphical representation of periodic table, referred as „Circular form of Periodic table‟. The advantages of circular form of periodic table over other representations are discussed along with a brief discussion on history of periodic tables. 1. Introduction The profoundness of inherent symmetry in nature can be seen at different depths of atomic scales. Periodic table symbolizes one such elegant symmetry existing within the atomic structure of chemical elements. This so called „symmetry‟ within the atomic structures has been widely studied from different prospects and over the last hundreds years more than 700 different graphical representations of Periodic tables have emerged [1]. Each graphical representation of chemical elements attempted to portray certain symmetries in form of columns, rows, spirals, dimensions etc. Out of all the graphical representations, the rectangular form of periodic table (also referred as Long form of periodic table or Modern periodic table) has gained wide acceptance. -

Quantum Theory of the Hydrogen Atom
Quantum Theory of the Hydrogen Atom Chemistry 35 Fall 2000 Balmer and the Hydrogen Spectrum n 1885: Johann Balmer, a Swiss schoolteacher, empirically deduced a formula which predicted the wavelengths of emission for Hydrogen: l (in Å) = 3645.6 x n2 for n = 3, 4, 5, 6 n2 -4 •Predicts the wavelengths of the 4 visible emission lines from Hydrogen (which are called the Balmer Series) •Implies that there is some underlying order in the atom that results in this deceptively simple equation. 2 1 The Bohr Atom n 1913: Niels Bohr uses quantum theory to explain the origin of the line spectrum of hydrogen 1. The electron in a hydrogen atom can exist only in discrete orbits 2. The orbits are circular paths about the nucleus at varying radii 3. Each orbit corresponds to a particular energy 4. Orbit energies increase with increasing radii 5. The lowest energy orbit is called the ground state 6. After absorbing energy, the e- jumps to a higher energy orbit (an excited state) 7. When the e- drops down to a lower energy orbit, the energy lost can be given off as a quantum of light 8. The energy of the photon emitted is equal to the difference in energies of the two orbits involved 3 Mohr Bohr n Mathematically, Bohr equated the two forces acting on the orbiting electron: coulombic attraction = centrifugal accelleration 2 2 2 -(Z/4peo)(e /r ) = m(v /r) n Rearranging and making the wild assumption: mvr = n(h/2p) n e- angular momentum can only have certain quantified values in whole multiples of h/2p 4 2 Hydrogen Energy Levels n Based on this model, Bohr arrived at a simple equation to calculate the electron energy levels in hydrogen: 2 En = -RH(1/n ) for n = 1, 2, 3, 4, . -

Power Sources Challenge
POWER SOURCES CHALLENGE FUSION PHYSICS! A CLEAN ENERGY Summary: What if we could harness the power of the Sun for energy here on Fusion reactions occur when two nuclei come together to form one Earth? What would it take to accomplish this feat? Is it possible? atom. The reaction that happens in the sun fuses two Hydrogen atoms together to produce Helium. It looks like this in a very simplified way: Many researchers including our Department of Energy scientists and H + H He + ENERGY. This energy can be calculated by the famous engineers are taking on this challenge! In fact, there is one DOE Laboratory Einstein equation, E = mc2. devoted to fusion physics and is committed to being at the forefront of the science of magnetic fusion energy. Each of the colliding hydrogen atoms is a different isotope of In order to understand a little more about fusion energy, you will learn about hydrogen, one deuterium and one the atom and how reactions at the atomic level produce energy. tritium. The difference in these isotopes is simply one neutron. Background: It all starts with plasma! If you need to learn more about plasma Deuterium has one proton and one physics, visit the Power Sources Challenge plasma activities. neutron, tritium has one proton and two neutrons. Look at the The Fusion Reaction that happens in the SUN looks like this: illustration—do you see how the mass of the products is less than the mass of the reactants? That is called a mass deficit and that difference in mass is converted into energy. -

Principal, Azimuthal and Magnetic Quantum Numbers and the Magnitude of Their Values
268 A Textbook of Physical Chemistry – Volume I Principal, Azimuthal and Magnetic Quantum Numbers and the Magnitude of Their Values The Schrodinger wave equation for hydrogen and hydrogen-like species in the polar coordinates can be written as: 1 휕 휕휓 1 휕 휕휓 1 휕2휓 8휋2휇 푍푒2 (406) [ (푟2 ) + (푆푖푛휃 ) + ] + (퐸 + ) 휓 = 0 푟2 휕푟 휕푟 푆푖푛휃 휕휃 휕휃 푆푖푛2휃 휕휙2 ℎ2 푟 After separating the variables present in the equation given above, the solution of the differential equation was found to be 휓푛,푙,푚(푟, 휃, 휙) = 푅푛,푙. 훩푙,푚. 훷푚 (407) 2푍푟 푘 (408) 3 푙 푘=푛−푙−1 (−1)푘+1[(푛 + 푙)!]2 ( ) 2푍 (푛 − 푙 − 1)! 푍푟 2푍푟 푛푎 √ 0 = ( ) [ 3] . exp (− ) . ( ) . ∑ 푛푎0 2푛{(푛 + 푙)!} 푛푎0 푛푎0 (푛 − 푙 − 1 − 푘)! (2푙 + 1 + 푘)! 푘! 푘=0 (2푙 + 1)(푙 − 푚)! 1 × √ . 푃푚(퐶표푠 휃) × √ 푒푖푚휙 2(푙 + 푚)! 푙 2휋 It is obvious that the solution of equation (406) contains three discrete (n, l, m) and three continuous (r, θ, ϕ) variables. In order to be a well-behaved function, there are some conditions over the values of discrete variables that must be followed i.e. boundary conditions. Therefore, we can conclude that principal (n), azimuthal (l) and magnetic (m) quantum numbers are obtained as a solution of the Schrodinger wave equation for hydrogen atom; and these quantum numbers are used to define various quantum mechanical states. In this section, we will discuss the properties and significance of all these three quantum numbers one by one. Principal Quantum Number The principal quantum number is denoted by the symbol n; and can have value 1, 2, 3, 4, 5…..∞. -

This Ubiquitous Carbon…
Engineering Physics Department Presents Dr. Cristian Contescu Senior Research Staff, Materials Science and Technology Division Oak Ridge National Laboratory This ubiquitous carbon… Abstract: After Stone Age, Bronze Age, and Iron Age, and after the Silicon Age of the informational revolution, the technologies of 21st century are marked by the ubiquitous presence of various forms of carbon allotropes. For a very long time, diamond and graphite were the only known carbon allotropes, but that has changed with the serendipitous discovery of fullerenes, carbon nanotubes, and graphene. Every ten or fifteen years scientists unveil new forms of carbons with new and perplexing properties, while computations suggest that the carbon’s family still has members unknown to us today. At a dramatically accelerated pace, new carbon forms find their place at the leading edge of scientific and technological innovations. At the same time traditional forms of carbon are being used in new and exciting applications that make our life safer, healthier, and more enjoyable. The 21st century may soon be recognized as the Age of Carbon forms. This educational talk will show how carbon, the fourth most abundant element in the Galaxy and the basis of life on Earth, was the engine of most important technological developments throughout the history of civilization. It will emphasize the ability of carbon atoms to generate a variety of mutual combinations and with many other chemical elements. These properties have placed carbon at the core of numerous inventions that define our civilization, while emerging new technologies open a rich path for value-added products in today’s market. -

The Chemical Origin of Behavior Is Rooted in Abiogenesis
Life 2012, 2, 313-322; doi:10.3390/life2040313 OPEN ACCESS life ISSN 2075-1729 www.mdpi.com/journal/life Article The Chemical Origin of Behavior is Rooted in Abiogenesis Brian C. Larson, R. Paul Jensen and Niles Lehman * Department of Chemistry, Portland State University, P.O. Box 751, Portland, OR 97207, USA; E-Mails: [email protected] (B.C.L.); [email protected] (R.P.J.) * Author to whom correspondence should be addressed; E-Mail: [email protected]; Tel.: +1-503-725-8769. Received: 6 August 2012; in revised form: 29 September 2012 / Accepted: 2 November 2012 / Published: 7 November 2012 Abstract: We describe the initial realization of behavior in the biosphere, which we term behavioral chemistry. If molecules are complex enough to attain a stochastic element to their structural conformation in such as a way as to radically affect their function in a biological (evolvable) setting, then they have the capacity to behave. This circumstance is described here as behavioral chemistry, unique in its definition from the colloquial chemical behavior. This transition between chemical behavior and behavioral chemistry need be explicit when discussing the root cause of behavior, which itself lies squarely at the origins of life and is the foundation of choice. RNA polymers of sufficient length meet the criteria for behavioral chemistry and therefore are capable of making a choice. Keywords: behavior; chemistry; free will; RNA; origins of life 1. Introduction Behavior is an integral feature of life. Behavior is manifest when a choice is possible, and a living entity responds to its environment in one of multiple possible ways. -

TEK 8.5C: Periodic Table
Name: Teacher: Pd. Date: TEK 8.5C: Periodic Table TEK 8.5C: Interpret the arrangement of the Periodic Table, including groups and periods, to explain how properties are used to classify elements. Elements and the Periodic Table An element is a substance that cannot be separated into simpler substances by physical or chemical means. An element is already in its simplest form. The smallest piece of an element that still has the properties of that element is called an atom. An element is a pure substance, containing only one kind of atom. The Periodic Table of Elements is a list of all the elements that have been discovered and named, with each element listed in its own element square. Elements are represented on the Periodic Table by a one or two letter symbol, and its name, atomic number and atomic mass. The Periodic Table & Atomic Structure The elements are listed on the Periodic Table in atomic number order, starting at the upper left corner and then moving from the left to right and top to bottom, just as the words of a paragraph are read. The element’s atomic number is based on the number of protons in each atom of that element. In electrically neutral atoms, the atomic number also represents the number of electrons in each atom of that element. For example, the atomic number for neon (Ne) is 10, which means that each atom of neon has 10 protons and 10 electrons. Magnesium (Mg) has an atomic number of 12, which means it has 12 protons and 12 electrons. -

Properties of Carbon the Atomic Element Carbon Has Very Diverse
Properties of Carbon The atomic element carbon has very diverse physical and chemical properties due to the nature of its bonding and atomic arrangement. fig. 1 Allotropes of Carbon Some allotropes of carbon: (a) diamond, (b) graphite, (c) lonsdaleite, (d–f) fullerenes (C60, C540, C70), (g) amorphous carbon, and (h) carbon nanotube. Carbon has several allotropes, or different forms in which it can exist. These allotropes include graphite and diamond, whose properties span a range of extremes. Despite carbon's ability to make 4 bonds and its presence in many compounds, it is highly unreactive under normal conditions. Carbon exists in 2 main isotopes: 12C and 13C. There are many other known isotopes, but they tend to be short-lived and have extremely short half-lives. Allotropes The different forms of a chemical element. Cabon is the chemical element with the symbol C and atomic number 6. As a member of group 14 on the periodic table, it is nonmetallic and tetravalent—making four electrons available to form covalent chemical bonds. Carbon has 6 protons and 6 Source URL: https://www.boundless.com/chemistry/nonmetallic-elements/carbon/properties-carbon/ Saylor URL: http://www.saylor.org/courses/chem102#6.1 Attributed to: Boundless www.saylor.org Page 1 of 2 neutrons, and has a standard atomic weight of 12.0107 amu. Its electron configuration is denoted as 1s22s22p2. It is a solid, and sublimes at 3,642 °C. It's oxidation state ranges from 4 to -4, and it has an electronegativity rating of 2.55 on the Pauling scale. Carbon has several allotropes, or different forms in which it exists. -

Chapter 10 – Chemical Reactions Notes
Chapter 8 – Chemical Reactions Notes Chemical Reactions: Chemical reactions are processes in which the atoms of one or more substances are rearranged to form different chemical compounds. How to tell if a chemical reaction has occurred (recap): Temperature changes that can’t be accounted for. o Exothermic reactions give off energy (as in fire). o Endothermic reactions absorb energy (as in a cold pack). Spontaneous color change. o This happens when things rust, when they rot, and when they burn. Appearance of a solid when two liquids are mixed. o This solid is called a precipitate. Formation of a gas / bubbling, as when vinegar and baking soda are mixed. Overall, the most important thing to remember is that a chemical reaction produces a whole new chemical compound. Just changing the way that something looks (breaking, melting, dissolving, etc) isn’t enough to qualify something as a chemical reaction! Balancing Equations Notes: Things to keep in mind when looking at the recipes for chemical reactions: 1) The stuff before the arrow is referred to as the “reactants” or “reagents”, and the stuff after the arrow is called the “products.” 2) The number of atoms of each element is the same on both sides of the arrow. Even though there may be different numbers of molecules, the number of atoms of each element needs to remain the same to obey the law of conservation of mass. 3) The numbers in front of the formulas tell you how many molecules or moles of each chemical are involved in the reaction. 4) Equations are nothing more than chemical recipes.