Sterol Compositions in Some Cucurbitaceae Vegetable Oils
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

ATP-Citrate Lyase Has an Essential Role in Cytosolic Acetyl-Coa Production in Arabidopsis Beth Leann Fatland Iowa State University
Iowa State University Capstones, Theses and Retrospective Theses and Dissertations Dissertations 2002 ATP-citrate lyase has an essential role in cytosolic acetyl-CoA production in Arabidopsis Beth LeAnn Fatland Iowa State University Follow this and additional works at: https://lib.dr.iastate.edu/rtd Part of the Molecular Biology Commons, and the Plant Sciences Commons Recommended Citation Fatland, Beth LeAnn, "ATP-citrate lyase has an essential role in cytosolic acetyl-CoA production in Arabidopsis " (2002). Retrospective Theses and Dissertations. 1218. https://lib.dr.iastate.edu/rtd/1218 This Dissertation is brought to you for free and open access by the Iowa State University Capstones, Theses and Dissertations at Iowa State University Digital Repository. It has been accepted for inclusion in Retrospective Theses and Dissertations by an authorized administrator of Iowa State University Digital Repository. For more information, please contact [email protected]. ATP-citrate lyase has an essential role in cytosolic acetyl-CoA production in Arabidopsis by Beth LeAnn Fatland A dissertation submitted to the graduate faculty in partial fulfillment of the requirements for the degree of DOCTOR OF PHILOSOPHY Major: Plant Physiology Program of Study Committee: Eve Syrkin Wurtele (Major Professor) James Colbert Harry Homer Basil Nikolau Martin Spalding Iowa State University Ames, Iowa 2002 UMI Number: 3158393 INFORMATION TO USERS The quality of this reproduction is dependent upon the quality of the copy submitted. Broken or indistinct print, colored or poor quality illustrations and photographs, print bleed-through, substandard margins, and improper alignment can adversely affect reproduction. In the unlikely event that the author did not send a complete manuscript and there are missing pages, these will be noted. -

Overexpression of Key Sterol Pathway Enzymes in Two Model Marine
bioRxiv preprint doi: https://doi.org/10.1101/2020.07.30.228171; this version posted July 30, 2020. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC-ND 4.0 International license. 1 Overexpression of key sterol pathway enzymes in two model 2 marine diatoms alters sterol profiles in Phaeodactylum 3 tricornutum 4 Ana Cristina Jaramillo-Madrid1, Raffaela Abbriano1, Justin Ashworth1, Michele Fabris1,2, 5 Peter J. Ralph1 6 1Climate Change Cluster, University of Technology Sydney, Australia, NSW 2007 7 2CSIRO Synthetic Biology Future Science Platform, GPO Box 2583, Brisbane, QLD 4001 8 Abstract 9 Sterols are a class of triterpenoid molecules with diverse functional roles in eukaryotic cells, 10 including intracellular signaling and regulation of cell membrane fluidity. Diatoms are a 11 dominant eukaryotic phytoplankton group that produce a wide diversity of sterol compounds. 12 The enzymes 3-hydroxy-3-methyl glutaryl CoA reductase (HMGR) and squalene epoxidase 13 (SQE) have been reported to be rate-limiting steps in sterol biosynthesis in other model 14 eukaryotes; however, the extent to which these enzymes regulate triterpenoid production in 15 diatoms is not known. To probe the role of these two metabolic nodes in the regulation of 16 sterol metabolic flux in diatoms, we independently over-expressed two versions of the native 17 HMGR and a conventional, heterologous SQE gene in the diatoms Thalassiosira pseudonana 18 and Phaeodactylum tricornutum. Overexpression of these key enzymes resulted in significant 19 differential accumulation of downstream sterol pathway intermediates in P. -

Nutritional Value, Applications, and Health Benefits
International Journal of Environmental Research and Public Health Review Sea Buckthorn in Plant Based Diets. An Analytical Approach of Sea Buckthorn Fruits Composition: Nutritional Value, Applications, and Health Benefits Anca-Mihaela Gâtlan * and Gheorghe Gutt Food Engineering Faculty, “S, tefan cel Mare” University, 720229 Suceava, Romania; g.gutt@fia.usv.ro * Correspondence: anca.gatlan@fia.usv.ro; Tel.: +40-747-532-695 Abstract: Current nutritional trends include plant-based diets as nutritional behavior of consumers who are increasingly concerned about a healthy lifestyle. Sea buckthorn (Hippophaë rhamnoides L.) is a plant with great virtues, containing more than 100 types of compounds. It is a plant with versatile properties, multiple economic advantages and a rich history, which still continues in natural medicine, and it is hence included in the daily diet by more and more people for the prevention and treatment of diet-related diseases. Its uniqueness is due to its chemical composition and the health beneficial properties that rise from its composition. This review is a detailed analytical picture of the current state of knowledge currently available regarding the Hippophaë plant, providing an overview of the qualities of sea buckthorn. This article summarizes data on sea buckthorn’s nutritional value, health beneficial properties, and its applications. Keywords: Hippophaë rhamnoides; sea buckthorn; plant-based diet; analytical characterization; nutri- Citation: Gâtlan, A.-M.; Gutt, G. Sea tional value; applications; health beneficial properties Buckthorn in Plant Based Diets. An Analytical Approach of Sea Buckthorn Fruits Composition: Nutritional Value, Applications, and Health Benefits. Int. J. Environ. Res. 1. Introduction Public Health 2021, 18, 8986. -

Metabolism of Cyclopropane Fatty Acids by Tetrahymena Pyriformis Najah M
Iowa State University Capstones, Theses and Retrospective Theses and Dissertations Dissertations 1973 Metabolism of cyclopropane fatty acids by Tetrahymena pyriformis Najah M. Al-Shathir Iowa State University Follow this and additional works at: https://lib.dr.iastate.edu/rtd Part of the Biochemistry Commons Recommended Citation Al-Shathir, Najah M., "Metabolism of cyclopropane fatty acids by Tetrahymena pyriformis " (1973). Retrospective Theses and Dissertations. 4989. https://lib.dr.iastate.edu/rtd/4989 This Dissertation is brought to you for free and open access by the Iowa State University Capstones, Theses and Dissertations at Iowa State University Digital Repository. It has been accepted for inclusion in Retrospective Theses and Dissertations by an authorized administrator of Iowa State University Digital Repository. For more information, please contact [email protected]. INFORMATION TO USERS This material was produced from a microfilm copy of the original document. While the most advanced technological means to photograph and reproduce this document have been used, the quality is heavily dspendent upon the quality of the original submitted. The following explanation of techniques is provided to help you understand markings or patterns which may appear on this reproduction. 1. The sign or "target" for pages apparently lacking from the document photographed is "Missing Page(s)". If it was possible to obtain the missing page(s) or section, they are spliced into the film along with adjacent pages. This may have necessitated cutting thru an image and duplicating adjacent pages to insure you complete continuity. 2. When an image on the film is obliterated with a large round black mark, it is an indication that the photographer suspected that the copy may have moved during exposure and thus cause a blurred image. -
Generate Metabolic Map Poster
Authors: Zheng Zhao, Delft University of Technology Marcel A. van den Broek, Delft University of Technology S. Aljoscha Wahl, Delft University of Technology Wilbert H. Heijne, DSM Biotechnology Center Roel A. Bovenberg, DSM Biotechnology Center Joseph J. Heijnen, Delft University of Technology An online version of this diagram is available at BioCyc.org. Biosynthetic pathways are positioned in the left of the cytoplasm, degradative pathways on the right, and reactions not assigned to any pathway are in the far right of the cytoplasm. Transporters and membrane proteins are shown on the membrane. Marco A. van den Berg, DSM Biotechnology Center Peter J.T. Verheijen, Delft University of Technology Periplasmic (where appropriate) and extracellular reactions and proteins may also be shown. Pathways are colored according to their cellular function. PchrCyc: Penicillium rubens Wisconsin 54-1255 Cellular Overview Connections between pathways are omitted for legibility. Liang Wu, DSM Biotechnology Center Walter M. van Gulik, Delft University of Technology L-quinate phosphate a sugar a sugar a sugar a sugar multidrug multidrug a dicarboxylate phosphate a proteinogenic 2+ 2+ + met met nicotinate Mg Mg a cation a cation K + L-fucose L-fucose L-quinate L-quinate L-quinate ammonium UDP ammonium ammonium H O pro met amino acid a sugar a sugar a sugar a sugar a sugar a sugar a sugar a sugar a sugar a sugar a sugar K oxaloacetate L-carnitine L-carnitine L-carnitine 2 phosphate quinic acid brain-specific hypothetical hypothetical hypothetical hypothetical -

Manipulation by Tridemorph, a Systemic Fungicide, of The
Plant Physiol. (1983) 71, 756-762 0032-0889/83/71/0756/07/$00.50/0 Manipulation by Tridemorph, a Systemic Fungicide, of the Sterol Composition of Maize Leaves and Roots' Received for publication June 16, 1982 and in revised form December 1, 1982 MICHELE BLADOCHA AND PIERRE BENVENISTE Laboratoire de Biochimie Vegetale, Institut de Botanique, 67083-Strasbourg Cedex, France ABSTRACT work deals with the action of Tridemorph, a systemic fungicide (19, 20), on sterol biosynthesis in Zea mays plants. Tridemorph The roots of maize (Zea mays L. var LG11) seedlings were watered with has been shown to block the CO2 (26) in suspension cultures of a solution of Tridemorph (2,6-dhmethyl-N-tridecyl-morphollne), a systemic Rubus fruticosus cells, resulting in an accumulation of 9i,B19- fungicide, for 3 to 4 weeks from the onset of germination. Very few A5- cyclopropyl sterols. The results reported here show that, when sterols, the major sterols ofthe control, were detected in the treated plnts, Tridemorph is given to maize plants, 9,8,19-cyclopropyl sterols and 9,8,19-cyckpropyl sterols accumulated dramatically in both roots and accumulate dramatically in roots and leaves, and the usual A5- leaves. A5-sterols were also found when low concentrations of the drug sterols (stigmasterol and sitosterol) are lost accordingly. were used. The time course of the accumulation of the new sterols has been studied in plants treated with various concentrations (1-20 milligrams per liter) of Tridemorph. We found that: (a) cycloeucalenol-obtusifoliol MATERIALS AND METHODS isomerase, an enzyme opening the cyclopropane ring ofcyclopropyl sterols, was strongly Inibited by the drug; and (b) the drug dhfused readily from Plant Material. -

A Different Function for a Member of an Ancient and Highly Conserved Cytochrome P450 Family: from Essential Sterols to Plant Defense
A different function for a member of an ancient and highly conserved cytochrome P450 family: From essential sterols to plant defense Xiaoquan Qi*†, Saleha Bakht*, Bo Qin*, Mike Leggett‡, Andrew Hemmings§, Fred Mellon¶, John Eagles¶, Daniele Werck-Reichhartʈ, Hubert Schallerʈ, Agnes Lesotʈ, Rachel Melton*, and Anne Osbourn*,** *Department of Metabolic Biology, John Innes Centre, Norwich NR4 7UH, United Kingdom; ‡Institute of Grassland and Environmental Research, Aberystwyth SY23 3EB, Wales, United Kingdom; §School of Biological Sciences and School of Chemical Sciences and Pharmacy, University of East Anglia, Norwich NR4 7TJ, United Kingdom; ¶Institute of Food Research, Norwich NR4 7UA, United Kingdom; ʈInstitute of Plant Molecular Biology, Centre National de la Recherche´Scientifique–Unite Propre de Recherche´2357, Universite Louis Pasteur, 67000 Strasbourg, France; and †Laboratory of Photosynthesis and Environmental Molecular Physiology, Institute of Botany, Chinese Academy of Sciences, Nanxincun 20, Fragrance Hill, Beijing 100093, China Edited by Klaus Hahlbrock, Max Planck Institute for Plant Breeding Research, Cologne, Germany, and approved October 10, 2006 (received for review September 7, 2006) CYP51 sterol demethylases are the only cytochrome P450 enzymes compromised in disease resistance to a range of fungal patho- with a conserved function across the animal, fungal, and plant gens, demonstrating that avenacins confer broad-spectrum pro- kingdoms (in the synthesis of essential sterols). These highly tection against microbial attack (5). These experiments have conserved enzymes, which are important targets for cholesterol- provided direct evidence for a role for preformed antimicrobial lowering drugs, antifungal agents, and herbicides, are regarded as compounds in plant defense. the most ancient member cytochrome P450 family. Here we Avenacins are synthesized from the isoprenoid pathway and present a report of a CYP51 enzyme that has acquired a different share a common biogenetic origin with sterols, the two pathways function. -

Inhibition of Ergosterol Biosynthesis by Etaconazole in Ustilago Maydis
Inhibition of Ergosterol Biosynthesis by Etaconazole in Ustilago maydis Edith Ebert, John Gaudin, Wolfgang Muecke, Klaus Ramsteiner, and Christian Vogel Agricultural Division, CIBA-GEIGY Limited, CH-4002 Basel, Switzerland Hermann Führer Central Research Laboratories, CIBA-GEIGY Limited, CH-4002 Basel, Switzerland Z. Naturforsch. 38 c, 2 8 -3 4 (1983); received July 22, 1982 Fungal Lipids, Ergosterol, Inhibition, Etaconazole, Fungicide The triazole fungicide etaconazole (CGA64 251) interferes with the ergosterol biosynthesis in Ustilago maydis by inhibiting the C-14 demethylation of the sterol nucleus. During the late log growth phase of U. maydis a novel endogenous sterol metabolite (14x-methyl-ergosta-8,24(28)- dien-3/?,6a-diol) was discovered and analyzed, which accumulates under the influence of the fungicide. The structure of this metabolite points to a hydroxylation-dehydration mechanism for the introduction of the double bond at C-5 during the ergosterol biosynthesis. Introduction investigated, as well as the accumulation of the various sterol intermediates of ergosterol biosyn In the past decade a series of substances have thesis studied after an inhibition time of only a few been discovered that inhibit the biosynthesis of hours. It was of interest for us to follow up the ergosterol. Some of them have been developed as appearance and accumulation of the various sterol fungicides and introduced to practical application. intermediates of ergosterol biosynthesis throughout They include various structural classes, such as the entire growth phase of the fungus under the piperazines (triforine), pyridines (S-1358), pyrimi influence of etaconazole. dines (fenarimol, triarimol), triazoles (triadimefon), and others [1]. More recently, further analogues of one of these classes of compounds, namely the M ethods triazoles, have been developed as plant protection /. -

Organ-Specificity of Sterol and Triterpene Accumulation in Arabidopsis Thaliana 2 3 4 B
bioRxiv preprint doi: https://doi.org/10.1101/2020.03.23.004358; this version posted March 25, 2020. The copyright holder for this preprint (which was not certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission. 1 Organ-specificity of sterol and triterpene accumulation in Arabidopsis thaliana 2 3 4 B. Markus Lange1,*, Brenton C. Poirier1,3, Iris Lange1, Richard Schumaker1,4, 5 and Rigoberto Rios-Estepa2 6 7 8 1 Institute of Biological Chemistry and M.J. Murdock Metabolomics Laboratory, Washington State 9 University, PO Box 646340, Pullman, WA 99164-6340, USA 10 11 2 Grupo de Bioprocesos, Departamento de Ingeniería Química, Universidad de Antioquia, Calle 12 70 No. 52-21, Medellín, Colombia 13 14 3 Current address: Elo Life Sciences, 5 Laboratory Drive, Research Triangle Park, NC 27709, USA 15 16 4 Current address: Alma Rosa Winery, 180-C Industrial Way, Buellton, CA 93427, USA 17 18 19 20 * Corresponding author: 21 B. M. Lange, Institute of Biological Chemistry and M.J. Murdock Metabolomics Laboratory, 22 Washington State University, PO Box 646340, Pullman, WA 99164-6340, USA; Tel.: 509-335-3794; 23 Fax: 509-335-7643; e-Mail: [email protected] 24 25 26 Running title: 27 Arabidopsis sterols and triterpenes 28 29 Keywords: 30 Arabidopsis; kinetic mathematical model; metabolic engineering; sterol; triterpene 31 1 bioRxiv preprint doi: https://doi.org/10.1101/2020.03.23.004358; this version posted March 25, 2020. The copyright holder for this preprint (which was not certified by peer review) is the author/funder. -
On the Occurrence of Cytochrome P450 in Viruses
On the occurrence of cytochrome P450 in viruses David C. Lamba,b,1, Alec H. Follmerc,1, Jared V. Goldstonea,1, David R. Nelsond, Andrew G. Warrilowb, Claire L. Priceb, Marie Y. Truee, Steven L. Kellyb, Thomas L. Poulosc,e,f, and John J. Stegemana,2 aBiology Department, Woods Hole Oceanographic Institution, Woods Hole, MA 02543; bInstitute of Life Science, Swansea University Medical School, Swansea University, Swansea, SA2 8PP Wales, United Kingdom; cDepartment of Chemistry, University of California, Irvine, CA 92697-3900; dDepartment of Microbiology, Immunology and Biochemistry, University of Tennessee Health Science Center, Memphis, TN 38163; eDepartment of Pharmaceutical Sciences, University of California, Irvine, CA 92697-3900; and fDepartment of Molecular Biology and Biochemistry, University of California, Irvine, CA 92697-3900 Edited by Michael A. Marletta, University of California, Berkeley, CA, and approved May 8, 2019 (received for review February 7, 2019) Genes encoding cytochrome P450 (CYP; P450) enzymes occur A core set of genes involved in viral replication and lysis is most widely in the Archaea, Bacteria, and Eukarya, where they play often conserved in these viruses (10), yet most genes in the giant important roles in metabolism of endogenous regulatory mole- viruses (70–90%) do not have any obvious homolog in existing cules and exogenous chemicals. We now report that genes for virus databases. multiple and unique P450s occur commonly in giant viruses in the Strikingly, many of the giant viruses possess genes coding for Mimiviridae Pandoraviridae , , and other families in the proposed proteins typically involved in cellular processes, including protein order Megavirales. P450 genes were also identified in a herpesvi- translation, DNA repair, and eukaryotic metabolic pathways Ranid herpesvirus 3 Mycobacterium rus ( ) and a phage ( phage previously thought not to occur in viruses (17). -

Generated by SRI International Pathway Tools Version 19.5 on Wed
Authors: Chuan Wang Peifen Zhang Pascal Schlapfer Taehyong Kim AraCyc: Arabidopsis thaliana col Cellular Overview Seung Yon Rhee 2+ 2+ + 2+ + 2+ 2+ Cd 2+ Cu 2+ + 2+ 2+ + H Cu H 2+ Cu Cu + Cd Cd H Cd Cu H 2+ 2+ Mn K Fe Fe cadmium/zinc- copper- AT2G18960.1 AT5G21930.1 AT1G20260.1 AT1G10130.1 AT5G44790.1 AT1G63440.1 AT5G55630.1 transporting AT4G30120.1 transporting AT2G19110 AT1G76030 AT4G30110 AT4G33520.2 AT4G38510 AT4G19680.2 AT4G19690.2 ATPase ATPase + 2+ + 2+ 2+ 2+ 2+ + 2+ 2+ + H Cu H 2+ Cu Cu + 2+ Cd 2+ Cd H Cd Cu H 2+ 2+ Mn K Cd Cu Fe Fe SECONDARY METABOLITES DEGRADATION phytate degradation I δ isoleucine AMINO ACIDS BIOSYNTHESIS β biosynthesis I glutamine glutamate histidine biosynthesis -alanine threonine superpathway superpathway of arginine biosynthesis II (acetyl cycle) superpathway of phenylalanine L-N -acetylornithine biosynthesis proline biosynthesis III superpathway of leucine, valine, biosynthesis II of aspartate and and tyrosine biosynthesis ALDEHYDE DEGRADATION and isoleucine biosynthesis biosynthesis III biosynthesis V biosynthesis isoleucine and asparagine biosynthesis valine biosynthesis TCA CYCLE TCA cycle variation V (plant) Ins(1,2,3,4,5,6)P 6 oxaloacetate N-acetylglutamyl- methylglyoxal PRPP propanoate pyruvate oxaloacetate phosphate chorismate pro glt degradation I methylglyoxal thr pyruvate gln 2-oxoglutarate oxaloacetate pyruvate thr d1-pyrroline- degradation III aspartate ATP-phosphoribosyl chorismate mutase: pyruvate 3-phytase: acetolactate pyruvate, transferase: AtATP-PRT1 butyrate- aspartate transaminase: -
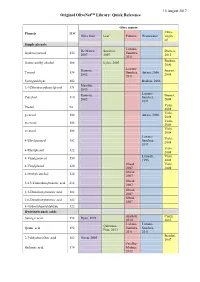
Olivenet Library Quick Reference Download
15 August 2017 Original OliveNet™ Library: Quick Reference Olive matrix Phenols MW Extra- Olive fruit Leaf Pomace Wastewater virgin oil Simple phenols Lozano- De Marco, Savarese, Dierkes, Hydroxytyrosol 154 Sanchez, 2007 2007 2012 2011 Boskou, Homovanillyl alcohol 168 Eyles, 2007 2006 Lozano- Romero, Suarez, Tyrosol 138 Sanchez, Artajo, 2006 2002 2008 2011 Syringaldehyde 182 Boskou, 2006 Marsilio, 3,4-Dihydroxyphenylglycol 171 2005 Lozano- Romero, Brenes, Catechol 110 Sanchez, 2002 2004 2011 Vichi, Phenol 94 2008 Vichi, p-cresol 108 Artajo, 2006 2008 Vichi, m-cresol 108 2008 Vichi, o-cresol 108 2008 Lozano- Vichi, 4-Ethylguaiacol 152 Sanchez, 2008 2011 Vichi, 4-Ethylphenol 122 2008 Limiroli, Vichi, 4-Vinylguaiacol 150 1996 2008 Obeid, Vichi, 4-Vinylphenol 120 2007 2008 Obeid, 4-Methylcatechol 124 2007 Obeid, 3,4,5-Trimethoxybenzoic acid 212 2007 Obeid, 3,4-Dimethoxybenzoic acid 182 2007 Obeid, 2,6-Dimethoxybenzoic acid 182 2007 4-Hydroxybenzaldehyde 122 Hydroxybenzoic acids Alu'datt, Cioffi, Syringic acid 198 Ryan, 1999 2010 2010 Lozano- Lozano- Quirantes- Quinic acid 192 Sanchez, Sanchez, Pine, 2013 2011 2011 Bendini, 2,3-dihydrocaffeic acid 182 Owen, 2003 2007 Peralbo- Shikimic acid 174 Molina, 2012 Peralbo- McDonald, Cioffi, Gallic acid 170 Molina, 2001 2010 2012 De la Peralbo- Romero, De Marco, Torre- Vanillic acid 168 Molina, 2002 2007 Carbot, 2012 2005 Phloretic acid 166 Owen, 2003 Alu'datt, Protocatechuic acid 154 Boskou, 2006 2010 Bendini, Gentisic acid 154 2007 Quirantes- Alu'datt, Caponio 4-hydroxybenzoic acid 138 Boskou, 2006