The Light Microscope, the Unstained Organism Is Silver, to Render
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Phagocytosis of Borrelia Burgdorferi, the Lyme Disease Spirochete, Potentiates Innate Immune Activation and Induces Apoptosis in Human Monocytes Adriana R
University of Connecticut OpenCommons@UConn UCHC Articles - Research University of Connecticut Health Center Research 1-2008 Phagocytosis of Borrelia burgdorferi, the Lyme Disease Spirochete, Potentiates Innate Immune Activation and Induces Apoptosis in Human Monocytes Adriana R. Cruz University of Connecticut School of Medicine and Dentistry Meagan W. Moore University of Connecticut School of Medicine and Dentistry Carson J. La Vake University of Connecticut School of Medicine and Dentistry Christian H. Eggers University of Connecticut School of Medicine and Dentistry Juan C. Salazar University of Connecticut School of Medicine and Dentistry See next page for additional authors Follow this and additional works at: https://opencommons.uconn.edu/uchcres_articles Part of the Medicine and Health Sciences Commons Recommended Citation Cruz, Adriana R.; Moore, Meagan W.; La Vake, Carson J.; Eggers, Christian H.; Salazar, Juan C.; and Radolf, Justin D., "Phagocytosis of Borrelia burgdorferi, the Lyme Disease Spirochete, Potentiates Innate Immune Activation and Induces Apoptosis in Human Monocytes" (2008). UCHC Articles - Research. 182. https://opencommons.uconn.edu/uchcres_articles/182 Authors Adriana R. Cruz, Meagan W. Moore, Carson J. La Vake, Christian H. Eggers, Juan C. Salazar, and Justin D. Radolf This article is available at OpenCommons@UConn: https://opencommons.uconn.edu/uchcres_articles/182 INFECTION AND IMMUNITY, Jan. 2008, p. 56–70 Vol. 76, No. 1 0019-9567/08/$08.00ϩ0 doi:10.1128/IAI.01039-07 Copyright © 2008, American Society for Microbiology. All Rights Reserved. Phagocytosis of Borrelia burgdorferi, the Lyme Disease Spirochete, Potentiates Innate Immune Activation and Induces Apoptosis in Human Monocytesᰔ Adriana R. Cruz,1†‡ Meagan W. Moore,1† Carson J. -

Borrelia Burgdorferi and Treponema Pallidum: a Comparison of Functional Genomics, Environmental Adaptations, and Pathogenic Mechanisms
PERSPECTIVE SERIES Bacterial polymorphisms Martin J. Blaser and James M. Musser, Series Editors Borrelia burgdorferi and Treponema pallidum: a comparison of functional genomics, environmental adaptations, and pathogenic mechanisms Stephen F. Porcella and Tom G. Schwan Laboratory of Human Bacterial Pathogenesis, Rocky Mountain Laboratories, National Institute of Allergy and Infectious Diseases, NIH, Hamilton, Montana, USA Address correspondence to: Tom G. Schwan, Rocky Mountain Laboratories, 903 South 4th Street, Hamilton, Montana 59840, USA. Phone: (406) 363-9250; Fax: (406) 363-9445; E-mail: [email protected]. Spirochetes are a diverse group of bacteria found in (6–8). Here, we compare the biology and genomes of soil, deep in marine sediments, commensal in the gut these two spirochetal pathogens with reference to their of termites and other arthropods, or obligate parasites different host associations and modes of transmission. of vertebrates. Two pathogenic spirochetes that are the focus of this perspective are Borrelia burgdorferi sensu Genomic structure lato, a causative agent of Lyme disease, and Treponema A striking difference between B. burgdorferi and T. pal- pallidum subspecies pallidum, the agent of venereal lidum is their total genomic structure. Although both syphilis. Although these organisms are bound togeth- pathogens have small genomes, compared with many er by ancient ancestry and similar morphology (Figure well known bacteria such as Escherichia coli and Mycobac- 1), as well as by the protean nature of the infections terium tuberculosis, the genomic structure of B. burgdorferi they cause, many differences exist in their life cycles, environmental adaptations, and impact on human health and behavior. The specific mechanisms con- tributing to multisystem disease and persistent, long- term infections caused by both organisms in spite of significant immune responses are not yet understood. -

Introduction to Bacteriology and Bacterial Structure/Function
INTRODUCTION TO BACTERIOLOGY AND BACTERIAL STRUCTURE/FUNCTION LEARNING OBJECTIVES To describe historical landmarks of medical microbiology To describe Koch’s Postulates To describe the characteristic structures and chemical nature of cellular constituents that distinguish eukaryotic and prokaryotic cells To describe chemical, structural, and functional components of the bacterial cytoplasmic and outer membranes, cell wall and surface appendages To name the general structures, and polymers that make up bacterial cell walls To explain the differences between gram negative and gram positive cells To describe the chemical composition, function and serological classification as H antigen of bacterial flagella and how they differ from flagella of eucaryotic cells To describe the chemical composition and function of pili To explain the unique chemical composition of bacterial spores To list medically relevant bacteria that form spores To explain the function of spores in terms of chemical and heat resistance To describe characteristics of different types of membrane transport To describe the exact cellular location and serological classification as O antigen of Lipopolysaccharide (LPS) To explain how the structure of LPS confers antigenic specificity and toxicity To describe the exact cellular location of Lipid A To explain the term endotoxin in terms of its chemical composition and location in bacterial cells INTRODUCTION TO BACTERIOLOGY 1. Two main threads in the history of bacteriology: 1) the natural history of bacteria and 2) the contagious nature of infectious diseases, were united in the latter half of the 19th century. During that period many of the bacteria that cause human disease were identified and characterized. 2. Individual bacteria were first observed microscopically by Antony van Leeuwenhoek at the end of the 17th century. -

Treponema Pallidum, the Syphilis Spirochete: Making a Living As a Stealth Pathogen
HHS Public Access Author manuscript Author ManuscriptAuthor Manuscript Author Nat Rev Manuscript Author Microbiol. Author Manuscript Author manuscript; available in PMC 2017 June 01. Published in final edited form as: Nat Rev Microbiol. 2016 December ; 14(12): 744–759. doi:10.1038/nrmicro.2016.141. Treponema pallidum, the syphilis spirochete: making a living as a stealth pathogen Justin D. Radolf1, Ranjit K. Deka2, Arvind Anand3, David Šmajs4, Michael V. Norgard5, and X. Frank Yang6 1Departments of Medicine, Pediatrics, Genetics and Genomic Science, Molecular Biology and Biophysics, and Immunology, UConn Health, Farmington, CT, USA 2Department of Microbiology, The University of Texas Southwestern Medical Center, Dallas, TX, USA 3Department of Medicine, UConn Health, Farmington, CT, USA 4Department of Biology, Faculty of Medicine, Masaryk University, Brno, Czech Republic 5Department of Microbiology, The University of Texas Southwestern Medical Center, Dallas, TX, USA 6Department of Microbiology and Immunology, Indiana University School of Medicine, Indianapolis, IN Abstract The last two decades have seen a worldwide resurgence in infections caused by Treponema pallidum subsp. pallidum, the syphilis spirochete. The syphilis spirochete’s well-recognized capacity for early dissemination and immune evasion has earned it the designation ‘the stealth pathogen’. Despite the many hurdles to studying syphilis pathogenesis, most notably the inability to culture and to genetically manipulate T. pallidum, in recent years, considerable progress has been made in elucidating the structural, physiologic, and regulatory facets of stealth pathogenicity. In this Review, we integrate this eclectic body of information to garner fresh insights into the highly successful parasitic lifestyles of the syphilis spirochete and related pathogenic treponemes. Pathogenic treponemes cause venereal syphilis, yaws, endemic syphilis, and pinta—multi- stage, infections that, although similar, can be differentiated based on clinical, epidemiologic, and geographic criteria1,2. -

WHO GUIDELINES for the Treatment of Treponema Pallidum (Syphilis)
WHO GUIDELINES FOR THE Treatment of Treponema pallidum (syphilis) WHO GUIDELINES FOR THE Treatment of Treponema pallidum (syphilis) WHO Library Cataloguing-in-Publication Data WHO guidelines for the treatment of Treponema pallidum (syphilis). Contents: Web annex D: Evidence profiles and evidence-to-decision frameworks - Web annex E: Systematic reviews for syphilis guidelines - Web annex F: Summary of conflicts of interest 1.Syphilis – drug therapy. 2.Treponema pallidum. 3.Sexually Transmitted Diseases. 4.Guideline. I.World Health Organization. ISBN 978 92 4 154980 6 (NLM classification: WC 170) © World Health Organization 2016 All rights reserved. Publications of the World Health Organization are available on the WHO website (http://www.who.int) or can be purchased from WHO Press, World Health Organization, 20 Avenue Appia, 1211 Geneva 27, Switzerland (tel.: +41 22 791 3264; fax: +41 22 791 4857; email: [email protected]). Requests for permission to reproduce or translate WHO publications – whether for sale or for non-commercial distribution– should be addressed to WHO Press through the WHO website (http://www.who.int/about/licensing/ copyright_form/index.html). The designations employed and the presentation of the material in this publication do not imply the expression of any opinion whatsoever on the part of the World Health Organization concerning the legal status of any country, territory, city or area or of its authorities, or concerning the delimitation of its frontiers or boundaries. Dotted and dashed lines on maps represent approximate border lines for which there may not yet be full agreement. The mention of specific companies or of certain manufacturers’ products does not imply that they are endorsed or recommended by the World Health Organization in preference to others of a similar nature that are not mentioned. -

Morphology of Spirochaeta Myelophthora in Multiple Sclerosis
MORPHOLOGY OF SPIROCHAETA MYELOPHTHORA IN MULTIPLE SCLEROSIS GABRIEL STEINER, M.D. (Detroit, Mich.) In a recent paper (1) the findings of specific spirochetes in the brain of a newly examined subacute case of multiple sclerosis were reported and evaluated. The purpose of the present paper is to give a detailed description of these spiro chetes, their classification, their reproduction, and disintegration. Four cases of multiple sclerosis, including the case to be reported, elicited abundant numbers of specific spirochetes in the central nervous system to war rant the publication of this paper. Downloaded from 1. Further Evidence of Spirochetal Nature: It has been said that the reported spirochetes represent only spirochete-like structures of the tissue proper, such as reticulin fibrils, neurofibrils, or axis cylinders.* To disprove these objections the following experiments were done: Sections from brains of general paresis containing numerous spirochetes in the cortex and those from lungs in congenital syphilis were stained with my silver technique II (1), then desilverized with potassium permanganate and oxalic acid (A. J. Wilson (2)), http://jnen.oxfordjournals.org/ and restained for reticulin fibers (Wilder's method). They showed clearly the complete absence of spirochetes in these restained sections, at regions where many spirochetes were formerly seen. Reticulin fibrils were easily demonstrable. Such sections could be desilverized again and restained for spirochetes with positive results. In sections stained for axis cylin ders (Bielschowsky's axis cylinder method for paraffin sections), no spirochetes were seen in places where previously masses of treponemas were present. Desilverizing and restaining with technique II brought the spirochetes out again. -

Applications of Microscopy in Bacteriology
Microscopy Research, 2016, 4, 1-9 Published Online January 2016 in SciRes. http://www.scirp.org/journal/mr http://dx.doi.org/10.4236/mr.2016.41001 Applications of Microscopy in Bacteriology Mini Mishra1, Pratima Chauhan2* 1Centre of Environmental Studies, Department of Botany, University of Allahabad, Allahabad, India 2Department of Physics, University of Allahabad, Allahabad, India Received 28 September 2015; accepted 2 January 2016; published 5 January 2016 Copyright © 2016 by authors and Scientific Research Publishing Inc. This work is licensed under the Creative Commons Attribution International License (CC BY). http://creativecommons.org/licenses/by/4.0/ Abstract Bacteria are smallest primitive, simple, unicellular, prokaryotic and microscopic organisms. But these organisms cannot be studied with naked eyes because of their minute structure. Therefore in search for the information about the structure and composition of bacterial cells, cell biologist used light microscopes with a numerical aperture of 1.4 and using wavelength of 0.4 µm separa- tion. But there are still certain cellular structures that cannot be seen through naked eyes, and for them electron microscope is used. There are certain improved types of light microscope which can be incorporated to improve their resolving power. Hence microscopy is playing a crucial role in the field of bacteriology. Keywords AFM, SEM, TEM, Microscopy, Bacteriology 1. Introduction To get acquainted with the world of bacteria like small organisms, very effective and advanced technique is re- quired. The size of bacteria ranges between 0.5 - 5.0 micrometer in length; the smallest of them are members of mycoplasma which measures 0.3 micrometers [1]. -

Medical Bacteriology
LECTURE NOTES Degree and Diploma Programs For Environmental Health Students Medical Bacteriology Abilo Tadesse, Meseret Alem University of Gondar In collaboration with the Ethiopia Public Health Training Initiative, The Carter Center, the Ethiopia Ministry of Health, and the Ethiopia Ministry of Education September 2006 Funded under USAID Cooperative Agreement No. 663-A-00-00-0358-00. Produced in collaboration with the Ethiopia Public Health Training Initiative, The Carter Center, the Ethiopia Ministry of Health, and the Ethiopia Ministry of Education. Important Guidelines for Printing and Photocopying Limited permission is granted free of charge to print or photocopy all pages of this publication for educational, not-for-profit use by health care workers, students or faculty. All copies must retain all author credits and copyright notices included in the original document. Under no circumstances is it permissible to sell or distribute on a commercial basis, or to claim authorship of, copies of material reproduced from this publication. ©2006 by Abilo Tadesse, Meseret Alem All rights reserved. Except as expressly provided above, no part of this publication may be reproduced or transmitted in any form or by any means, electronic or mechanical, including photocopying, recording, or by any information storage and retrieval system, without written permission of the author or authors. This material is intended for educational use only by practicing health care workers or students and faculty in a health care field. PREFACE Text book on Medical Bacteriology for Medical Laboratory Technology students are not available as need, so this lecture note will alleviate the acute shortage of text books and reference materials on medical bacteriology. -

Laboratory Diagnostic Testing for Treponema Pallidum
Laboratory Diagnostic Testing for Treponema pallidum Expert Consultation Meeting Summary Report January 13‐15, 2009 Atlanta, GA This report was produced in cooperation with the Centers for Disease Control and Prevention. Laboratory Diagnostic Testing for Treponema pallidum Expert Consultation Meeting Summary Report January 13‐15, 2009 Atlanta, GA In the last decade there have been major changes and improvements in STD testing technologies. While these changes have created great opportunities for more rapid and accurate STD diagnosis, they may also create confusion when laboratories attempt to incorporate new technologies into the existing structure of their laboratory. With this in mind, the Centers for Disease Control and Prevention (CDC) and the Association of Public Health Laboratories (APHL) convened an expert panel to evaluate available information and produce recommendations for inclusion in the Guidelines for the Laboratory Diagnosis of Treponema pallidum in the United States. An in‐person meeting to formulate these recommendations was held on January 13‐15, 2009 on the CDC Roybal campus. The panel included public health laboratorians, STD researchers, STD clinicians, STD Program Directors and other STD program staff. Representatives from the Food and Drug Administration (FDA) and Centers for Medicare & Medicaid Services (CMS) were also in attendance. The target audience for these recommendations includes laboratory directors, laboratory staff, microbiologists, clinicians, epidemiologists, and disease control personnel. For several months prior to the in‐person consultation, these workgroups developed key questions and researched the current literature to ensure that any recommendations made were relevant and evidence based. Published studies compiled in Tables of Evidence provided a framework for group discussion addressing several key questions. -

History of the Department of Microbiology 1868 – 2009
June 2015 HISTORY OF THE DEPARTMENT OF MICROBIOLOGY 1868 – 2009 University of Illinois at Urbana-Champaign 1 A HISTORY OF THE DEPARTMENT OF MICROBIOLOGY 1868 – 2009 This 141 year history of the Department of Microbiology includes an article (Chapter 1), written and published in 1959 by the Department, which covers the period 1868 to 1959. I joined the Department in 1953, and my recounting of the Department’s history includes personal observations as well as anecdotes told to me by H. O. Halvorson and others. Later I realized what a unique experience it had been to join a first-class department, and I resolved to play a role in maintaining its research stature. Ralph Wolfe 2 Department of Microbiology History of the Headship: 1950 – 1959 Halvor Halvorson 1960 – 1963 Kim Atwood 1963 – 1972 Leon Campbell 1972 – 1982 Ralph DeMoss 1982 – 1987 Samuel Kaplan 1987 – 1990 Jordan Konisky 1990 – 1991 Ralph Wolfe (Acting Head) 1991 – 1997 Charles Miller 1997 – 2002 John Cronan 2003 – 2004 Jeffrey Gardner (Acting Head) 2005 – Present John Cronan 3 Organization of the History of the Department In Chapters 2 to 6 the data are divided into Academic Decades, each containing the following sections: Section I, an overview of the decade; Section II, some events for each year of the decade; Section III, a summary of the research interests, honors received, publications, and invited off-campus lectures or seminars for each faculty member. These data have been obtained from the annual reports of the faculty submitted to the departmental secretary. 4 CHAPTER 1 1868 – 1959 During this time period the name of the Department was Department of Bacteriology (Anecdotes by Ralph Wolfe) A SHORT HISTORY OF THE DEPARTMENT OF BACTERIOLOGY H. -
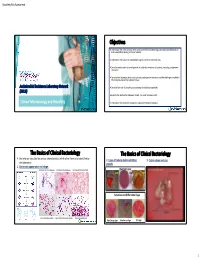
Objectives the Basics of Clinical Bacteriology the Basics of Clinical
Biosafety Risk Assessment Objectives Understand the most common tests used by the clinical bacteriology laboratory for identification and susceptibility testing of clinical isolates. Understand the classes of antimicrobial agents and their potential uses. Describe mechanisms for development of antibiotic resistance in bacteria, including carbapenem resistance. Describe the laboratory tests used to detect carbapenem resistance and the challenges involved in the interpretation of the laboratory data. Antimicrobial Resistance Laboratory Network Describe the role of biosafety in protecting the healthcare provider. (ARLN) Explain the relationship between hazard, risk, and risk assessment. Clinical Microbiology and Biosafety Understand the Antibiotic Resistance Laboratory Network initiative. The Basics of Clinical Bacteriology The Basics of Clinical Bacteriology Bacteria are classified by various characteristics which allow them to be identified by 2. Types of culture media exhibiting 3. Colony shape and size: the laboratory: growth: 1. Gram stain appearance and shape: Gram Positive Cocci in chains (purple) Gram Positive Diplococci (purple) Gram Negative Diplococci (red/pink) Nutrient Agar BAP BAP BAP Selective and Differential Agar Gram Negative Rods(red/pink) Gram Positive Cocci in Clusters (purple) Gram Positive Rods (purple) MacConkey Agar MacConkey Agar XLD Agar 1 Biosafety Risk Assessment The Basics of Clinical Bacteriology The Basics of Clinical Bacteriology 4. Atmospheric requirements for bacterial growth: 5. Organism Identification: Spot tests – rapid biochemical tests which can be used to rule in/out various groups of organisms Catalase: Ability to breakdown H2O2 Oxidase: Presence of cytochrome oxidase CO2 – Neisseria spp., Haemophilus spp., Streptococcus pneumonia 2 Microaerophilic ( reduced O ) – Campylobacter spp. (+) Staphylococcus spp. (=) Streptococcus spp. Pseudomonas spp. E. coli (Enterobacteriaceae) Anaerobic (lack of O2) – Clostridium difficile The Basics of Clinical Bacteriology The Basics of Clinical Bacteriology 5. -

Prevotella Intermedia
The principles of identification of oral anaerobic pathogens Dr. Edit Urbán © by author Department of Clinical Microbiology, Faculty of Medicine ESCMID Online University of Lecture Szeged, Hungary Library Oral Microbiological Ecology Portrait of Antonie van Leeuwenhoek (1632–1723) by Jan Verkolje Leeuwenhook in 1683-realized, that the film accumulated on the surface of the teeth contained diverse structural elements: bacteria Several hundred of different© bacteria,by author fungi and protozoans can live in the oral cavity When these organisms adhere to some surface they form an organizedESCMID mass called Online dental plaque Lecture or biofilm Library © by author ESCMID Online Lecture Library Gram-negative anaerobes Non-motile rods: Motile rods: Bacteriodaceae Selenomonas Prevotella Wolinella/Campylobacter Porphyromonas Treponema Bacteroides Mitsuokella Cocci: Veillonella Fusobacterium Leptotrichia © byCapnophyles: author Haemophilus A. actinomycetemcomitans ESCMID Online C. hominis, Lecture Eikenella Library Capnocytophaga Gram-positive anaerobes Rods: Cocci: Actinomyces Stomatococcus Propionibacterium Gemella Lactobacillus Peptostreptococcus Bifidobacterium Eubacterium Clostridium © by author Facultative: Streptococcus Rothia dentocariosa Micrococcus ESCMIDCorynebacterium Online LectureStaphylococcus Library © by author ESCMID Online Lecture Library Microbiology of periodontal disease The periodontium consist of gingiva, periodontial ligament, root cementerum and alveolar bone Bacteria cause virtually all forms of inflammatory