Oil of Catnip by Supercritical Fluid Extraction
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Ectoparasites of Free-Roaming Domestic Cats in the Central United States
Veterinary Parasitology 228 (2016) 17–22 Contents lists available at ScienceDirect Veterinary Parasitology journal homepage: www.elsevier.com/locate/vetpar Research paper Ectoparasites of free-roaming domestic cats in the central United States a b,1 a a,∗ Jennifer E. Thomas , Lesa Staubus , Jaime L. Goolsby , Mason V. Reichard a Department of Veterinary Pathobiology, Center for Veterinary Health Sciences, Oklahoma State University, 250 McElroy Hall Stillwater, OK 74078, USA b Department of Clinical Science, Center for Veterinary Health Sciences, Oklahoma State University, 1 Boren Veterinary Medical Teaching Hospital Stillwater, OK 74078, USA a r t i c l e i n f o a b s t r a c t Article history: Free-roaming domestic cat (Felis catus) populations serve as a valuable resource for studying ectoparasite Received 11 May 2016 prevalence. While they share a similar environment as owned cats, free-roaming cats do not receive rou- Received in revised form 27 July 2016 tine veterinary care or ectoparasiticide application, giving insight into parasite risks for owned animals. Accepted 29 July 2016 We examined up to 673 infested cats presented to a trap-neuter-return (TNR) clinic in the central United States. Ectoparasite prevalences on cats were as follows: fleas (71.6%), ticks (18.7%), Felicola subrostratus Keywords: (1.0%), Cheyletiella blakei (0.9%), and Otodectes cynotis (19.3%). Fleas, ticks, and O. cynotis were found in Cat all months sampled. A total of 1117 fleas were recovered from 322 infested cats. The predominate flea Feline recovered from cats was Ctenocephalides felis (97.2%) followed by Pulex spp. -

Think Twice Before You Declaw
This article first appeared in the Winter 2006 issue of Animal Behavior Consulting: Theory and Practice, a publication of the International Association of Animal Behavior Consultants, www.iaabc.org. Copyright 2006 The IAABC. Think Twice Before You Declaw The Itch to Scratch Cats do write. They don’t communicate Asking a cat never to scratch with a pen and paper or by using a com- is asking a cat not to act like puter keyboard. Instead, their prose is cat a cat. scratch—literally. They scratch to express their excitement and pleasure. They Most of us don’t mind that scratch to leave messages, both visual and cats scratch; what bothers us aromatic. (A cat’s paws have scent glands is where they scratch. But that leave smell-o-grams; we can’t read nearly all cats can be taught them, but other cats can.) where to scratch—and where not to. Kittens are Cats also scratch, not to sharpen their nails particularly easy to train, but to remove the worn-out sheaths from but it’s not that difficult to their claws. You see the results as little cres- teach the adults, either. The cent-moon shaped bits around scratching secret is to provide attractive areas. Scratching is good exercise, too. scratching alternatives to the sofa or stereo speakers and Scratching is normal behavior for cats. then teach the cat to use those alternatives. All cats scratch; it’s part of being a cat. Reality Check Just so you know, a typical declaw on a cat who goes outdoors, since de- (called an onychectomy) is an irre- clawed cats have been disarmed. -

Fish Technology Glossary
Glossary of Fish Technology Terms A Selection of Terms Compiled by Kevin J. Whittle and Peter Howgate Prepared under contract to the Fisheries Industries Division of the Food and Agriculture Organization of the United Nations 6 December 2000 Last updated: February 2002 Kevin J. Whittle 1 GLOSSARY OF FISH TECHNOLOGY TERMS [Words highlighted in bold in the text of an entry refer to another entry. Words in parenthesis are alternatives.] Abnormalities Attributes of the fish that are not found in the great majority of that kind of fish. For example: atypical shapes; overall or patchy discolorations of skin or of fillet; diseased conditions; atypical odours or flavours. Generally, the term should be used for peculiarities present in the fish at the time of capture or harvesting, or developing very soon after; peculiarities arising during processing should be considered as defects. Acetic acid Formal chemical name, ethanoic acid. An organic acid of formula CH3.COOH. It is the main component, 3-6%, other than water, of vinegar. Used in fish technology in preparation of marinades. Acid curing See Marinating Actomyosin A combination of the two main proteins, actin and myosin, present in all muscle tissues. Additive A chemical added to a food to affect its properties. Objectives of including additives in a product include: increased stability during storage; inhibition of growth of microorganisms or production of microbial toxins; prevention or reduction of formation of off-flavours; improved sensory properties, particularly colours and appearance, affecting acceptability to the consumer; improved properties related to preparation and processing of food, for example, ability to create stable foams or emulsions, or to stabilise or thicken sauces. -

Check List: for a Healthy Cat
CHECK LIST: FOR A HEALTHY CAT Congrats on your new pet! This welcome kit is a great reference for tips from Cascade Pet Hospital on how to keep your kitty healthy and happy. NECESSITIES OTHER SUGGESTED ITEMS • Premium Grade Food • Cat Treats for Training and Play, with or without Catnip • Bowls - Ceramic or Stainless Steel for Food & Water (Cats are Prone • Air-Tight Food Container & Scoop to Plastic Allergies) • Regular Grooming Program Cat • Litter Box & Litter (1 per Cat, Plus Bed 1 Additional in Multi-Cat Homes) • Change or Scoop Litter Daily • ID Tag & Microchip Safe • Books on Cat Care (breed specific) • Toys • Litter Genie • Pet Carrier (Appropriate for Size) • De-Shedding Tool • Stain Remover & Odor Eliminator (Do Not Use Ammonia) • Vertical Cat Tree • Flea Comb & Flea & Tick Control Products • Toothbrush Kit & Dental Aids (TD, CET Chews, etc.) • Bi-Yearly Exam with your Veterinarian DAILY PET CHECK: FOR A HEALTHY CAT MY PET • Is acting normal, active and happy. • Does not tire easily after moderate exercise. Does not have seizures or fainting episodes. • Has a normal appetite, with no significant weight change. Does not vomit or regurgitate food. • Has normal appearing bowel movements (firm, formed, mucus-free). Doesn’t scoot on the floor or chew under the tail excessively. • Has a full glossy coat with no missing hair, mats or excessive shedding. Doesn’t scratch, lick or chew excessively. • Has skin that is free of dry flakes, not greasy, and is odor-free. Is free from fleas, ticks or mites. • Has a body free from lumps and bumps. Has ears that are clean and odor-free. -

Δtb = M × Kb, Δtf = M × Kf
8.1HW Colligative Properties.doc Colligative Properties of Solvents Use the Equations given in your notes to solve the Colligative Property Questions. ΔTb = m × Kb, ΔTf = m × Kf Freezing Boiling K K Solvent Formula Point f b Point (°C) (°C/m) (°C/m) (°C) Water H2O 0.000 100.000 1.858 0.521 Acetic acid HC2H3O2 16.60 118.5 3.59 3.08 Benzene C6H6 5.455 80.2 5.065 2.61 Camphor C10H16O 179.5 ... 40 ... Carbon disulfide CS2 ... 46.3 ... 2.40 Cyclohexane C6H12 6.55 80.74 20.0 2.79 Ethanol C2H5OH ... 78.3 ... 1.07 1. Which solvent’s freezing point is depressed the most by the addition of a solute? This is determined by the Freezing Point Depression constant, Kf. The substance with the highest value for Kf will be affected the most. This would be Camphor with a constant of 40. 2. Which solvent’s freezing point is depressed the least by the addition of a solute? By the same logic as above, the substance with the lowest value for Kf will be affected the least. This is water. Certainly the case could be made that Carbon disulfide and Ethanol are affected the least as they do not have a constant. 3. Which solvent’s boiling point is elevated the least by the addition of a solute? Water 4. Which solvent’s boiling point is elevated the most by the addition of a solute? Acetic Acid 5. How does Kf relate to Kb? Kf > Kb (fill in the blank) The freezing point constant is always greater. -

Pet Care Tips for Cats
Pet Care Tips for cats What you’ll need to know to keep your companion feline happy and healthy . Backgroun d Cats were domesticated sometime between 4,000 and 8,000 years ago, in Africa and the Middle East. Small wild cats started hanging out where humans stored their grain. When humans saw cats up close and personal, they began to admire felines for their beauty and grace. There are many different breeds of cats -- from the hairless Sphinx and the fluffy Persian to the silvery spotted Egyptian Mau . But the most popular felines of all are non-pedigree —that includes brown tabbies, black-and-orange tortoiseshells, all-black cats with long hair, striped cats with white socks and everything in between . Cost When you first get your cat, you’ll need to spend about $25 for a litter box, $10 for a collar, and $30 for a carrier. Food runs about $170 a year, plus $50 annually for toys and treats, $175 annually for litter and an average of $150 for veterinary care every year. Note: Make sure you have all your supplies (see our checklist) before you bring your new pet home. Basic Care Feeding - An adult cat should be fed one large or two or three smaller meals each day . - Kittens from 6 to 12 weeks must eat four times a day . - Kittens from three to six months need to be fed three times a day . You can either feed specific meals, throwing away any leftover canned food after 30 minutes, or keep dry food available at all times. -

Disrupting Crystalline Order to Restore Superfluidity
Abteilung Kommunikation und Öffentlichkeitsarbeit Referat Medien- und Öffentlichkeitsarbeit Tel. +49 40 42838-2968 Fax +49 40 42838-2449 E-Mail: [email protected] 12. Oktober 2018 Pressedienst 57/18 Disrupting crystalline order to restore superfluidity When we put water in a freezer, water molecules crystallize and form ice. This change from one phase of matter to another is called a phase transition. While this transition, and countless others that occur in nature, typically takes place at the same fixed conditions, such as the freezing point, one can ask how it can be influenced in a controlled way. We are all familiar with such control of the freezing transition, as it is an essential ingredient in the art of making a sorbet or a slushy. To make a cold and refreshing slushy with the perfect consistency, constant mixing of the liquid is needed. For example, a slush machine with constantly rotating blades helps prevent water molecules from crystalizing and turning the slushy into a solid block of ice. Imagine now controlling quantum matter in this same way. Rather than forming a normal liquid, like a melted slushy under the sun for too long, quantum matter can form a superfluid. This mysterious and counterintuitive form of matter was first observed in liquid helium at very low temperatures, less than 2 Kelvin above absolute zero. The helium atoms have a strong tendency to form a crystal, like the water molecules in a slushy, and this restricts the superfluid state of helium to very low temperatures and low pressures. But what if you could turn on the blades in your slush machine for quantum matter? What if you could disrupt the crystalline order so that the superfluid could flow freely even at temperatures and pressures where it usually does not? This is indeed the idea that was demonstrated by a team of scientists led by Ludwig Mathey and Andreas Hemmerich from the University of Hamburg. -

Physical Changes
How Matter Changes By Cindy Grigg Changes in matter happen around you every day. Some changes make matter look different. Other changes make one kind of matter become another kind of matter. When you scrunch a sheet of paper up into a ball, it is still paper. It only changed shape. You can cut a large, rectangular piece of paper into many small triangles. It changed shape and size, but it is still paper. These kinds of changes are called physical changes. Physical changes are changes in the way matter looks. Changes in size and shape, like the changes in the cut pieces of paper, are physical changes. Physical changes are changes in the size, shape, state, or appearance of matter. Another kind of physical change happens when matter changes from one state to another state. When water freezes and makes ice, it is still water. It has only changed its state of matter from a liquid to a solid. It has changed its appearance and shape, but it is still water. You can change the ice back into water by letting it melt. Matter looks different when it changes states, but it stays the same kind of matter. Solids like ice can change into liquids. Heat speeds up the moving particles in ice. The particles move apart. Heat melts ice and changes it to liquid water. Metals can be changed from a solid to a liquid state also. Metals must be heated to a high temperature to melt. Melting is changing from a solid state to a liquid state. -

Catnip Anyone? by Dawn Pettinelli, Uconn Home & Garden Education Center
Catnip Anyone? By Dawn Pettinelli, UConn Home & Garden Education Center Most plants we grow, indoors or out, are either useful to us, like vegetables, or attractive additions to our gardens and landscapes. One plant your pet cat will thank you for growing is catnip (Nepeta cataria). Catnip is a perennial member of the mint family native to parts of Asia and Europe. Like most mints, it is fairly easy to grow. If anything, mints are rather boisterous plants, always looking to expand their range, especially given their preferred conditions of sun and plenty of moisture. They can be restrained to some degree by growing them in partly shaded, drier parts of the garden. In the case of catnip, given a sunny window, it is perfectly happy to be grown as a houseplant, which is how I grow it for our inside cat. The scalloped-edged, pointy leaves are an attractive medium green and slightly hairy on the undersides. Stems are characteristically square. If grown outside, plants usually bloom once during the summer. The flowers are small and white with purple spots. Like all mints, they are attractive to honeybees and other pollinators. Catnip is easily grown from seed and will self-seed if the flowers are left to mature on the plant so remove them after blooming if this is not a desirable trait. A cat’s response to catnip may vary but can include rubbing or rolling on the plant, sniffing, licking, chewing on the leaves, vocalizing, drooling as well as other crazy antics. It’s not just domestic felines that find catnip alluring; big cats too are drawn to this aromatic herb. -

A Possible Force Mechanism Associated with the Freezing of Water in Porous Materials
A Possible Force Mechanism Associated with the Freezing of Water in Porous Materials LORNE W. GOLD Division of Building Research, National Research Council, Ottawa, Canada The thermodynamic equilibrium conditions for a water-ice interface in a pore of a porous medium are derived. It is found that, taking the geometry and physical dimensions of the pore into consideration, a positive pressure must develop between the ice and the solid in order for equilibrium to be maintained when the temperature at the freezing plane is depressed. A simple model utilizing this mecha• nism exhibits the same properties as a frost-heaving soil. The in• fluence of air in the pores is briefly discussed. #WHEN WATER is present in porous materials and is allowed to freeze, a force may be developed which is not directly related to the expansion that occurs when water changes to ice. Although many observations have been made on this phenomenon, par• ticularly in the field of soil mechanics, a satisfactory explanation of the origin of this force has not appeared. Experiments to date indicate how the magnitude of this force depends on certain physical characteristics of the system. The following are some of the facts observed during experiments that a theory on the origin of this force must explain: 1. During the freezing process, the freezing plane may remain stationary while the ice phase continues to grow. When this occurs, the frozen portion is displaced rela• tive to the unfrozen and a layer of ice, often called an ice lens is formed. The dis• placement may be microscopic or it may amount to several inches. -
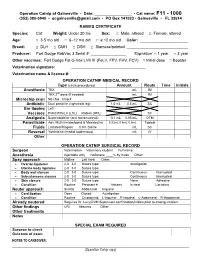
Operation Catnip Medical Records
Operation Catnip of Gainesville • Date: _________________ • Cat name: F11 - 1000 (352) 380-0940 • [email protected] • PO Box 141023 • Gainesville • FL 32614 RABIES CERTIFICATE Species: Cat Weight: Under 20 lbs Sex: □ Male, altered □ Female, altered Age: □ 3-5 mo old □ 6–12 mo old □ ≥ 12 mo old Color: ______________________ Breed: □ DLH □ DMH □ DSH □ Siamese/pointed ____________________________ Producer: Fort Dodge RabVac 3 Serial #: ________________ Expiration: □ 1 year □ 3 year Other vaccines: Fort Dodge Fel-O-Vax LVK III (FeLV, FPV, FHV, FCV) □ Initial dose □ Booster Veterinarian signature: __________________________________ Veterinarian name & license #: __________________________________ OPERATION CATNIP MEDICAL RECORD Type (circle procedures) Amount Route Time Initials Anesthesia TKX mL IM TKX 2nd dose (if needed) mL IM Microchip scan No chip Chip # Antibiotic Dual penicillin (right front leg) 1.0 mL 0.5 mL SC Ear tipping Left Vaccines FVRCP/FeLV (LHL) Rabies (RHL) SC Analgesia Buprenorphine (oral transmucosal) 0.1 mL 0.05 mL OTM Parasiticide Adv. Multi (Imidacloprid & Moxidectin) 0.23mL 0.4mL 0.8mL Topical Fluids Lactated Ringers 0.9% Saline mL SC Reversal Yohimbine (medial saphenous) mL IV Other OPERATION CATNIP SURGICAL RECORD Surgeon Veterinarian Veterinary student Full name: Anesthesia Injectable only Isoflurane ____% by mask Other: Spay approach Midline Left flank Other: Ovarian ligatures 2-0 3-0 Suture type: Autoligation Uterine body ligatures 2-0 3-0 Suture type: Body wall closure 2-0 3-0 Suture type: Continuous Interrupted Subcutaneous closure 2-0 3-0 Suture type: Continuous Interrupted Skin closure 2-0 3-0 Suture type: None Adhesive Condition Routine Pregnant #_______ fetuses In heat Lactating Neuter approach Scrotal Abdominal Inguinal Cord ligation Open Closed Autoligation Condition Routine Cryptorchid: L-Inguinal R-Inguinal L-Abdominal R-Abdominal Already neutered Requires Dr. -

Understanding Freezing Behavior in Nano-Porous Materials: Free
ÍÒÖ×ØÒÒ ÖÞÒ ÚÓÖ Ò ÆÒÓ¹Ô ÓÖÓÙ× ÅØÖÐ× Ö ÒÖ Ý ËÑÙÐØÓÒ ËØÙ× Ò ÜÔ ÖÑÒØ ××ÖØØÓÒ ÈÖ×ÒØ ØÓ Ø ÙÐØÝ Ó Ø Ö ÙØ Ë Ó ÓÐ Ó ÓÖÒÐÐ ÍÒÚÖ×ØÝ Ò ÈÖØÐ ÙЬÐÐÑÒØ Ó Ø ÊÕÙÖÑÒØ× ÓÖ Ø Ö Ó Ó ØÓÖ Ó È ÐÓ×ÓÔ Ý Ý ÊÚ ÊÖ×ÒÒ ÙÙ×Ø ¾¼¼¼ ÊÚ ÊÖ×ÒÒ ¾¼¼¼ ÄÄ ÊÁÀÌË ÊËÊÎ ÍÒÖ×ØÒÒ ÖÞÒ ÚÓÖ Ò ÆÒÓ¹Ô ÓÖÓÙ× ÅØÖÐ× Ö ÒÖÝ ËÑÙÐØÓÒ ËØÙ× Ò ÜÔ ÖÑÒØ ÊÚ ÊÖ×ÒÒ¸ Ⱥº ÓÖÒÐÐ ÍÒÚÖ×ØÝ ¾¼¼¼ ÍÒÖ×ØÒÒ Ô× ÚÓÖ Ò ÓÒ¬Ò ×Ý×ØÑ× × ØÖÑÒÓÙ× Ô ÓØÒØÐ Ò ÑÔÖÓÚÒ Ø Æ ÓÚÖØÝ Ó ×ÔÖØÓÒ ÔÖÓ ××× ØØ Ù× Ô ÓÖÓÙ× Ñ¹ ØÖÐ× Ð ØÚØ Ö ÓÒ׸ ÓÒØÖÓÐÐ Ð××׸ ×Ð ÜÖÓÐ× ² ÖÓÐ׸ Ö ÓÒ ÖÓÐ× Ò ÞÓÐØ׺ Ì «Ø× Ó Ø ÖÙ ÑÒ×ÓÒÐØÝ Ò ÒÒ ÒÖ¹ Ø ÒØÖØÓÒ× Ù ØÓ Ø ÔÓÖÓÙ× ×ÙÖ Ú ÑÔ ÓÖØÒØ ÓÒ×ÕÙÒ× ØØ Ò ÓÒÐÝ ÔØÙÖ Ý ÑÓÐÙÐÖ ÐÚÐ ÑÓ ÐÒº ÆÓÚÐ Ô× ØÖÒ×ØÓÒ× Ò Ö×ÙÐØ ´ÔÐÐÖÝ ÓÒÒ×ØÓÒ Ò ÕÙ×¹ÓÒ¹ÑÒ×ÓÒÐ ×Ý×ØÑ× Ò ÓÖÒØØÓÒÐ ÓÖÖÒ ØÖÒ×ØÓÒ× Ò ÕÙ×¹ØÛÓ¹ÑÒ×ÓÒÐ ×Ý×ØÑ×µ ØØ ÓØÒ Ö Ø Ù× Ó Ø Ö¹ ÓÛÒ Ó ÑÖÓ×ÓÔ ÕÙØÓÒ× Ð ÃÐÚÒ Ò ×¹ÌÓÑ×ÓÒ ÕÙØÓÒ× × ÓÒ Ð××Ð ØÖÑÓ ÝÒÑ׺ ÁÒ Ø× ÛÓÖ¸ Ø Ó Ù× ÓÒ Ö ÒÖÝ ÑØÓ × ÓÒ ÄÒÙ ØÓÖÝ Û Û ÚÐÓÔ Ò ÔÔÐØÓÒ× Ó Ø× ØÓÖÝ ØÓ ÙÒÖ×ØÒ Ø ÖÓÛÒ Ó Ø ÑÖÓ×ÓÔ ÕÙØÓÒ׸ ÚÐÓÔÒ ÐÓÐ Ô× ÖÑ׸ Ò ÖØÖÞÒ ÜØ Ô×׺ Ì Ö×ÙÐØ× Ó ÓÙÖ ÜÔ ÖÑÒØÐ ×ØÙ× Ö Ð×Ó ×Ö ¸ ØØ ÔÖÓÚ ×ÙÔÔ ÓÖØÒ ÚÒ ÓÖ ÓÙÖ ÑÓ ÐÒ «ÓÖØ׺ ÓÖÔÐ Ë Ø ÓÖÒ Ò Ò Ñ ÑÐÝ Ò ×ÓÙØÖÒ ÁÒ¸ Á Û× ÒØÖ×Ø Ò Ö×Ö ×Ò Ø Ý× Á Ò³Ø ÚÒ ÖÑÑÖº Á ÓÙÒ ÑÝ×Ð ´ÖØÖ ÓÖØÙÒØÐÝ ÓÖ Ñµ ×ØÙÝÒ ÑÐ ÒÒÖÒ Ò Ø ÁÒÒ ÁÒ×ØØÙØ Ó ÌÒÓÐÓÝ Ø ÅÖ׺ ÙÖÒ ÑÝ Ö×ÑÒ ÝÖ Á Û× ×ØÖÙ Ý Ø ÖÐÞØÓÒ ØØ Á Ñ ÒÓ ÐÓÒÖ Ø ÒÓÛ¹ÐÐ Ô Ö×ÓÒ Ò ×Ó Óи ØØ Ø ÛÓÖÐ × ÙÐÐ Ó ÔÝ×Ð ´Ò Ñе ÔÒÓÑÒ ØØ Á ÖÐÝ ÒÛ ÓÙغ Ø Ø ÒÒÒ Ó ÑÝÂÙÒÓÖÝÖ¸ Á ×ØÖØ ØÓ ÚÐÓÔ Ò ÒØÒ× ×ÒØÓÒ ÓÖ ×ØØ×ØÐ ÑÒ× Ò Ò ÑÝ ÑÒ ØÓ ÕÙØ ÑÐ ÒÒÖÒ Ò Ø ÙÔ ÔÝ×׺ ÁØ Û× Ô× ÛÖ Á Û× ÑÒ Ø ÓÒØÒØ× Ó ÑÒÝ Ó Ó× ÓÒ ×ØØ×ØÐ ÔÝ×׸ ÛÒ Á Ñ ÖÓ×× Ø ÓÓ ÅÓÐÙÐÖ ÌÖÑÓ ÝÒÑ× Ó ÐÙ È× ÕÙÐÖ Ý ÂÓÒ ÈÖÙ×ÒØÞº Ì× Û× ØÙÖÒÒ