Bacterial Toxin-Antitoxin Proteins and Induced Dormancy" (2016)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
FROM DNA to BEER Date Lesson Plan: Acquired and Passive Immunity Class Period
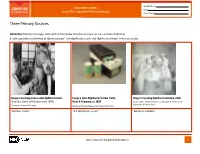
Student Name FROM DNA TO BEER Date Lesson Plan: Acquired and Passive Immunity Class Period Three Primary Sources Instruction: Examine the images and read their titles below. Based on what you can learn and infer from them: 1. Write your inferred definition of “diphtheria toxin,” “anti-diphtheritic serum,” and “diphtheria antitoxin” in the table below. Image 1. Injecting a horse with diphtheria toxin, Image 2. Anti-Diphtheritic Serum, Parke, Image 3. Injecting diphtheria antitoxin, 1895 New York City Health Department, 1940s Davis & Company, ca. 1898 Courtesy The Historical Medical Library of The College of Physicians of Philadelphia Courtesy Library of Congress Courtesy National Museum of American History “diphtheria toxin”: “anti-diphtheritic serum”: “diphtheria antitoxin”: http://www.nlm.nih.gov/fromdnatobeer 1 Student Name FROM DNA TO BEER Date Lesson Plan: Acquired and Passive Immunity Class Period Three Primary Sources 2. Describe or draw how the three images may be related. http://www.nlm.nih.gov/fromdnatobeer 2 FROM DNA TO BEER Lesson Plan: Acquired and Passive Immunity Teacher’s Three Primary Sources Instruction: Examine the images and read their titles below. Based on what you can learn and infer from them: 1. Write your inferred definition of “diphtheria toxin,” “anti-diphtheritic serum,” and “diphtheria antitoxin” in the table below. Image 1. Injecting a horse with diphtheria toxin, Image 2. Anti-Diphtheritic Serum, Parke, Image 3. Injecting diphtheria antitoxin, 1895 New York City Health Department, 1940s Davis & Company, -

Persistent Virus and Addiction Modules: an Engine of Symbiosis
UC Irvine UC Irvine Previously Published Works Title Persistent virus and addiction modules: an engine of symbiosis. Permalink https://escholarship.org/uc/item/5ck1g026 Journal Current opinion in microbiology, 31 ISSN 1369-5274 Author Villarreal, Luis P Publication Date 2016-06-01 DOI 10.1016/j.mib.2016.03.005 Peer reviewed eScholarship.org Powered by the California Digital Library University of California Available online at www.sciencedirect.com ScienceDirect Persistent virus and addiction modules: an engine of symbiosis Luis P Villarreal The giant DNA viruses are highly prevalent and have a particular host would occasionally survive but still retain a bit of affinity for the lytic infection of unicellular eukaryotic host. The the selfish virus DNA. Thus although parasitic selfish giant viruses can also be infected by inhibitory virophage which (virus-like) information is common in the genomes of all can provide lysis protection to their host. The combined life forms, its presence was explained as mostly defective protective and destructive action of such viruses can define a remnants of past plague sweeps that provides no func- general model (PD) of virus-mediated host survival. Here, I tional benefit to the host (e.g. junk). Until recently, this present a general model for role such viruses play in the explanation seemed satisfactory. In the last twenty years, evolution of host symbiosis. By considering how virus mixtures however, various observation-based developments have can participate in addiction modules, I provide a functional compelled us to re-evaluate this stance. Both comparative explanation for persistence of virus derived genetic ‘junk’ in genomics and metagenomics (sequencing habitats) has their host genomic habitats. -

Srna Antitoxins: More Than One Way to Repress a Toxin
Toxins 2014, 6, 2310-2335; doi:10.3390/toxins6082310 OPEN ACCESS toxins ISSN 2072-6651 www.mdpi.com/journal/toxins Review sRNA Antitoxins: More than One Way to Repress a Toxin Jia Wen and Elizabeth M. Fozo * Department of Microbiology, University of Tennessee, M409 Walters Life Sciences, Knoxville, TN 37996, USA; E-Mail: [email protected] * Author to whom correspondence should be addressed; E-Mail: [email protected]; Tel.: +1-865-974-4028; Fax: +1-865-974-4007. Received: 30 June 2014; in revised form: 15 July 2014 / Accepted: 17 July 2014 / Published: 4 August 2014 Abstract: Bacterial toxin-antitoxin loci consist of two genes: one encodes a potentially toxic protein, and the second, an antitoxin to repress its function or expression. The antitoxin can either be an RNA or a protein. For type I and type III loci, the antitoxins are RNAs; however, they have very different modes of action. Type I antitoxins repress toxin protein expression through interacting with the toxin mRNA, thereby targeting the mRNA for degradation or preventing its translation or both; type III antitoxins directly bind to the toxin protein, sequestering it. Along with these two very different modes of action for the antitoxin, there are differences in the functions of the toxin proteins and the mobility of these loci between species. Within this review, we discuss the major differences as to how the RNAs repress toxin activity, the potential consequences for utilizing different regulatory strategies, as well as the confirmed and potential biological roles for these loci across bacterial species. Keywords: type I toxin-antitoxin; type III toxin-antitoxin; small RNA; small peptide 1. -

Mechanisms of Plasmid Stable Maintenance with Special Focus on Plasmid Addiction Systems
Vol. 48 No. 4/2001 1003–1023 QUARTERLY Review Mechanisms of plasmid stable maintenance with special focus on plasmid addiction systems. ½ Urszula Zielenkiewicz and Piotr Ceg³owski Institute of Biochemistry and Biophysics, Polish Academy of Sciences Received: 5 November, 2001, accepted: 24 November, 2001 Key words: plasmid addiction, post-segregational killing, partition; multimer resolution The stable inheritance of bacterial plasmids is achieved by a number of different mechanisms. Among them are resolution of plasmid oligomers into monomers, active plasmid partitioning into dividing cells and selective killing of plasmid-free segre- gants. A special focus is given to the last mechanism. It involves a stable toxin and an unstable antidote. The antidotes neutralize their cognate toxins or prevent their syn- thesis. The different decay rates of the toxins and the antidotes underlie molecular mechanisms of toxin activation in plasmid-free cells. By eliminating of plasmid-free cells from the population of plasmid-bearing ones the toxin-antidote couples therefore act as plasmid addiction systems. Plasmids are separate, autonomous genetic burgdorferi, Fraser et al., 1997; Bacillus cereus, elements present in a cell independently of Carlson & Kolstø, 1994). It is commonly ac- chromosomes. Most plasmids are small: from cepted that plasmid genes do not encode infor- several to 100 kb, but sometimes they are so mation indispensable for the functioning of large that using the size criteria their distinc- the host cell. However, plasmids specify nu- tion from the chromosome is difficult (e.g. in merous features advantageous for the host in Vibrio cholerae, Yamaichi et al., 1999; in specific environments, such as resistance to Rhizobium meliloti, Honeycutt et al., 1993). -

(ACIP) General Best Guidance for Immunization
Appendix 1: Glossary Adverse event. An undesirable medical condition that occurs following vaccination which might be truly caused by a vaccine, or it might be pure coincidence. Adverse reaction. An undesirable medical condition that has been demonstrated to be caused by a vaccine. Evidence for the causal relation is usually obtained through randomized clinical trials, controlled epidemiologic studies, isolation of the vaccine strain from the pathogenic site, or recurrence of the condition with repeated vaccination (i.e., rechallenge); synonyms include side effect and adverse effect. Adjuvant. A vaccine component distinct from the antigen that enhances the immune response to the antigen. Antitoxin. A solution of antibodies against a toxin. Antitoxin can be derived from either human (e.g., tetanus immune globulin) or animal (usually equine) sources (e.g., diphtheria and botulism antitoxin). Antitoxins are used to confer passive immunity and for treatment. Hyperimmune globulin (specific). Special preparations obtained from blood plasma from donor pools preselected for a high antibody content against a specific antigen (e.g., hepatitis B immune globulin, varicella-zoster immune globulin, rabies immune globulin, tetanus immune globulin, vaccinia immune globulin, cytomegalovirus immune globulin, botulism immune globulin). Immune globulin. A sterile solution containing antibodies, which are usually obtained from human blood. It is obtained by cold ethanol fractionation of large pools of blood plasma and contains 15%-18% protein. Intended for intramuscular administration, immune globulin is primarily indicated for routine maintenance of immunity among certain immunodeficient persons and for passive protection against measles and hepatitis A. General Best Practice Guidelines for Immunization: Appendix 1: Glossary 189 Immunobiologic. Antigenic substances (e.g., vaccines and toxoids) or antibody- containing preparations (e.g., globulins and antitoxins) from human or animal donors. -

Identification and Characterization of a Novel Toxin–Antitoxin Module From
FEBS Letters 581 (2007) 1727–1734 Identification and characterization of a novel toxin–antitoxin module from Bacillus anthracis Shivangi Agarwala, Shivani Agarwalb, Rakesh Bhatnagara,* a School of Biotechnology, Jawaharlal Nehru University, New Delhi-110067, India b Gene Regulation Laboratory, School of Biotechnology, Jawaharlal Nehru University, New Delhi-110067, India Received 10 November 2006; revised 3 March 2007; accepted 20 March 2007 Available online 30 March 2007 Edited by Judit Ova´di (95–135 aa) [9]. When the bacterium looses the plasmid during Abstract Comparative genome analysis of Bacillus anthracis revealed a pair of linked genes encoding pemK (K, killer protein) a segregational event, the degradation of antitoxin by cellular and pemI (I, inhibitory protein) homologous to pem loci of other proteases renders the toxin free to execute its lethal effect. organisms. Expression of PemK in Escherichia coli and Bacillus Therefore, these modules have been implicated in maintaining anthracis was bacteriostatic whereas the concomitant expression the stability of extra-chromosomal elements in the host ensur- of PemI reversed the growth arrest. PemK expression effectively ing propagation of only plasmid-inherited population. The dif- inhibited protein synthesis with no significant effect on DNA rep- ferent decay rate of these toxins and antitoxins has been lication. Coexpression and interaction of these proteins con- envisioned to be the molecular basis of toxin activation in plas- firmed it to be a Type II addiction module. Thermal mid-free cells. Such a genetic unit has been termed as an denaturation analysis reflected poor conformational stability of ‘Addiction module’ because the cells become addicted to the PemI as compared to PemK. -

Genomic Analysis of Acidianus Hospitalis W1 a Host for Studying Crenarchaeal Virus and Plasmid Life Cycles
Extremophiles (2011) 15:487–497 DOI 10.1007/s00792-011-0379-y ORIGINAL PAPER Genomic analysis of Acidianus hospitalis W1 a host for studying crenarchaeal virus and plasmid life cycles Xiao-Yan You • Chao Liu • Sheng-Yue Wang • Cheng-Ying Jiang • Shiraz A. Shah • David Prangishvili • Qunxin She • Shuang-Jiang Liu • Roger A. Garrett Received: 4 March 2011 / Accepted: 26 April 2011 / Published online: 24 May 2011 Ó The Author(s) 2011. This article is published with open access at Springerlink.com Abstract The Acidianus hospitalis W1 genome consists stress. Complex and partially defective CRISPR/Cas/Cmr of a minimally sized chromosome of about 2.13 Mb and a immune systems are present and interspersed with five conjugative plasmid pAH1 and it is a host for the model vapBC gene pairs. Remnants of integrated viral genomes filamentous lipothrixvirus AFV1. The chromosome carries and plasmids are located at five intron-less tRNA genes and three putative replication origins in conserved genomic several non-coding RNA genes are predicted that are con- regions and two large regions where non-essential genes are served in other Sulfolobus genomes. The putative metabolic clustered. Within these variable regions, a few orphan orfB pathways for sulphur metabolism show some significant and other elements of the IS200/607/605 family are con- differences from those proposed for other Acidianus and centrated with a novel class of MITE-like repeat elements. Sulfolobus species. The small and relatively stable genome There are also 26 highly diverse vapBC antitoxin–toxin gene of A. hospitalis W1 renders it a promising candidate for pairs proposed to facilitate maintenance of local chromo- developing the first Acidianus genetic systems. -

Diphtheria. In: Epidemiology and Prevention of Vaccine
Diphtheria Anna M. Acosta, MD; Pedro L. Moro, MD, MPH; Susan Hariri, PhD; and Tejpratap S.P. Tiwari, MD Diphtheria is an acute, bacterial disease caused by toxin- producing strains of Corynebacterium diphtheriae. The name Diphtheria of the disease is derived from the Greek diphthera, meaning ● Described by Hippocrates in ‘leather hide.’ The disease was described in the 5th century 5th century BCE BCE by Hippocrates, and epidemics were described in the ● Epidemics described in 6th century AD by Aetius. The bacterium was first observed 6th century in diphtheritic membranes by Edwin Klebs in 1883 and cultivated by Friedrich Löffler in 1884. Beginning in the early ● Bacterium first observed in 1900s, prophylaxis was attempted with combinations of toxin 1883 and cultivated in 1884 and antitoxin. Diphtheria toxoid was developed in the early ● Diphtheria toxoid developed 7 1920s but was not widely used until the early 1930s. It was in 1920s incorporated with tetanus toxoid and pertussis vaccine and became routinely used in the 1940s. Corynebacterium diphtheria Corynebacterium diphtheriae ● Aerobic gram-positive bacillus C. diphtheriae is an aerobic, gram-positive bacillus. ● Toxin production occurs Toxin production (toxigenicity) occurs only when the when bacillus is infected bacillus is itself infected (lysogenized) by specific viruses by corynebacteriophages (corynebacteriophages) carrying the genetic information for carrying tox gene the toxin (tox gene). Diphtheria toxin causes the local and systemic manifestations of diphtheria. ● Four biotypes: gravis, intermedius, mitis, and belfanti C. diphtheriae has four biotypes: gravis, intermedius, mitis, ● All isolates should be tested and belfanti. All biotypes can become toxigenic and cause for toxigenicity severe disease. -

Bacterial Plasmid Addiction Systems and Their Implications for Antibiotic
PostDoc Journal Journal of Postdoctoral Research Vol. 5, No. 5, May 2017 www.postdocjournal.com Bacterial plasmid addiction systems and their implications for antibiotic drug development 1Jennifer Tsang, PhD 1 Beth Israel Deaconess Medical Center, Boston, MA 02115, USA *E-mail: [email protected] Abstract Bacteria frequently carry mobile genetic elements capable of being passed to other bacterial cells. An example of this is the transfer of plasmids (small, circular DNA molecules) that often contain antibiotic resistance genes from one bacterium to another. Plasmids have evolved mechanisms to ensure their survival through generations by employing plasmids segregation and replication machinery and plasmid addiction systems. Plasmid addiction systems utilize a post-segregational killing of cells that have not received a plasmid. In this review, the types of plasmid addiction systems are described as well as their prevalence in antibiotic resistant bacteria. Lastly, the possibility of targeting these plasmid addiction systems for the treatment of antibiotic resistant bacterial infections is explored. Keywords: plasmid, toxin-antitoxin system, plasmid addiction system, antibiotics, antimicrobial resistance Introduction Bacteria often carry mobile genetic elements divided between both daughter cells. However, capable of being passed from bacterium to low-copy number plasmids must use specific bacterium. One such element is the plasmid, a mechanisms to ensure that future cell small, circular, double-stranded DNA molecule. populations retain plasmids. For example, by Plasmids can be transferred to daughter cells random segregation a dividing cell with two upon replication (vertically transferred) or to copies of a plasmid has a 50% chance of both non-offspring cells (horizontally transferred). cells acquiring one plasmid and a 50% chance of Horizontal transfer of genetic material between one cell receiving both plasmids while the other bacterial cells can occur within the same or cell receives none. -

In Silico Analysis of Genetic Vapc Profiles from the Toxin-Antitoxin
microorganisms Article In Silico Analysis of Genetic VapC Profiles from the Toxin-Antitoxin Type II VapBC Modules among Pathogenic, Intermediate, and Non-Pathogenic Leptospira Alexandre P. Y. Lopes 1,* , Bruna O. P. Azevedo 1,2, Rebeca C. Emídio 1,2, Deborah K. Damiano 1, Ana L. T. O. Nascimento 1 and Giovana C. Barazzone 1 1 Laboratório Especial de Desenvolvimento de Vacinas—Centro de Biotecnologia, Instituto Butantan, Avenida Vital Brazil, 1500, 05503-900 São Paulo, Brazil; [email protected] (B.O.P.A.); [email protected] (R.C.E.); [email protected] (D.K.D.); [email protected] (A.L.T.O.N.); [email protected] (G.C.B.) 2 Programa de Pós-Graduação Interunidades em Biotecnologia, Instituto de Ciências Biomédicas, USP, Avenida Prof. Lineu Prestes, 1730, 05508-900 São Paulo, Brazil * Correspondence: [email protected]; Tel.: +55-11-2627-9475 Received: 29 January 2019; Accepted: 15 February 2019; Published: 20 February 2019 Abstract: Pathogenic Leptospira spp. is the etiological agent of leptospirosis. The high diversity among Leptospira species provides an array to look for important mediators involved in pathogenesis. Toxin-antitoxin (TA) systems represent an important survival mechanism on stress conditions. vapBC modules have been found in nearly one thousand genomes corresponding to about 40% of known TAs. In the present study, we investigated TA profiles of some strains of Leptospira using a TA database and compared them through protein alignment of VapC toxin sequences among Leptospira spp. genomes. Our analysis identified significant differences in the number of putative vapBC modules distributed in pathogenic, saprophytic, and intermediate strains: four in L. -

Current Medical Literature American J. Digestive Diseases, Fort Wayne, Ind. Am. J. Roentgenol. & Rad. Therapy, Springfield
American Journal of Public Health, New York Current Medical Literature 33:925-1042 (Aug.) 1943 National Board of Health 1879-1883. W. G. Smillie.—p. 925. Preventive Medicine Program of United States Army. J. S. Simmons. AMERICAN —p. 931. Home Methods and Their Effect on Quality The Association to of Drying Palatability, Cooking library lends periodicals members the Association and Nutritive Value of Foods. Esther L. 941. and to Batchelder.—p. individual subscribers in continental United States and Canada Blood and Malaria Parasite Staining with Eosin Azure Méthylène Blue for a of be borrowed a time. period three days. Three journals may at Methods. R. D. 948. Periodicals are available from 1933 to date. for issues Lillie.—p. Requests of Radio Habits of Attend earlier date cannot be filled. should be Listening Mothers Who Well Baby Clinics. Requests accompanied by L. Murray and C. E. 952. cover if one and 18 if three Margaret Turner.—p. stamps to postage (6 cents cents periodicals Surveys of Nutrition of Populations: 2. Protein Nutrition of Rural are requested). Periodicals the American Medical Asso¬ published by Population in Middle Tennessee. J. B. E. W. ciation are not available for but can be on Youmans, Patton, lending supplied purchase W. R. Ruth Kern and Ruth 955. order. as a rule are the of can be Sutton, Steinkamp.—p. Reprints property authors and Field for Health Education Personnel. Minnie obtained for permanent possession only from them. Experience Krueger Oed. —p. 965. Titles marked with an asterisk (*) are abstracted below. Dehydration Procedures and Their Effect on Vitamin Retention. -

Title: Identification of a Vapbc Toxin-Antitoxin System in a Thermophilic Bacterium Thermus Thermophilus HB27 Authors: Yuqi Fan
Title: Identification of a VapBC Toxin-Antitoxin System in a Thermophilic Bacterium Thermus thermophilus HB27 Authors: Yuqi Fan, Takayuki Hoshino and Akira Nakamura. Affiliation: Faculty of Life and Environmental Sciences, University of Tsukuba, 1-1-1 Tennodai, Tsukuba, Ibaraki 305-8572, Japan Corresponding author: Akira Nakamura, e-mail: [email protected], Tel: +81-29-853-6637, Fax: +81-29-853-6637 Abbreviations Dox doxycycline DTT dithiothreitol IPTG isopropyl-β-D-thiogalactopyranoside PAGE polyacrylamide gel electrophoresis PBS phosphate-buffered saline PMSF phenylmethanesulfonylfluoride RNase ribonuclease TA toxin-antitoxin 1 Abstract There are 12 putative toxin-antitoxin (TA) loci in the Thermus thermophilus HB27 genome, including four VapBC and three HicBA families. Expression of these seven putative toxin genes in Escherichia coli demonstrated that one putative VapC toxin TTC0125 and two putative HicA toxins, TTC1395 and TTC1705, inhibited cell growth, and co-expression with cognate antiotoxin genes rescued growth, indicating that these genes function as TA loci. In vitro analysis with the purified TTC0125 and total RNA/mRNA from E. coli and T. thermophilus showed that TTC0125 has RNase activity to rRNA and mRNA; this activity was inhibited by the addition of the purified TTC0126. Translation inhibition assays showed that TTC0125 inhibited protein synthesis by degrading mRNA but not by inactivating ribosomes. Amino acid substitutions of 14 predicted catalytic and conserved residues in VapC toxins to Ala or Asp in TTC0125 indicated that nine residues are important for its in vivo toxin activity and in vitro RNase activity. These data demonstrate that TTC0125-TTC0126 functions as a VapBC TA module and causes growth inhibition by degrading free RNA.