Chemistry's Table of Contents
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Timberlane High School Science Summer Reading Assignment
Timberlane High School Science Summer Reading Assignment: Course: Chemistry CCP Instructions Please read the following selection(s) from the book A Short History of Nearly Everything by Bill Bryson. Please provide written answers (short essay style) to the questions at the end of the reading •Questions adapted from Random House Publishing Inc. https://www.randomhouse.com/catalog/teachers_guides/9780767908184.pdf The written assignment is to be turned into your teacher by Friday Sept. 8th for potential full credit. Accepted until Sept 13th with 10% deduction in grade per day. Not accepted after Sept 13th. This is a graded assignment worth up to 3% of your quarter 1 grade. Grading Rubric: The writing will be assessed on the following 0 to 3 scales Each answer should be in a short essay style (minimum one paragraph). o 1: most answers are short one word answers. o 3: complete thoughts and sentences that fully convey the answers. Each answer should demonstrate evidence of reading to comprehension. o 1: answers indicate that the reading was not completed o 3: answers show clear comprehension of the reading Each answer should be correct, relevant to the topic, should strive for detail and completeness. o 1: answers are not relative to question or reading o 3: Answers demonstrate clear relevancy to passage and get to the heart of the rationale for question in relation to subject area. Each answer should refer to a specific statement or include a quote from the reading. o 1: the writing is vague, incomplete and contains little detail o 3: writing is detailed, complete and references specific statements or quotes from the reading passage. -

The History of Dunedin Income Growth Investment Trust
The History of Dunedin Income Growth Investment Trust PLC The first investment trust launched in Scotland, 1873 – 2018 Dunedin Income Growth Trust Investment Income Dunedin Foreword 1873 – 2018 This booklet, written for us by John Newlands, It is a particular pleasure for me, as Chairman of DIGIT describes the history of Dunedin Income Growth and as former employee of Robert Fleming & Co to be Investment Trust PLC, from its formation in Dundee able to write a foreword to this history. It was Robert in February 1873 through to the present day. Fleming’s vision that established the trust. The history Launched as The Scottish American Investment Trust, of the trust and its role in making professional “DIGIT”, as the Company is often known, was the first investment accessible is as relevant today as it investment trust formed in Scotland and has been was in the 1870s when the original prospectus was operating continuously for the last 145 years. published. I hope you will find this story of Scottish enterprise, endeavour and vision, and of investment Notwithstanding the Company’s long life, and the way over the past 145 years interesting and informative. in which it has evolved over the decades, the same The Board of DIGIT today are delighted that the ethos of investing in a diversified portfolio of high trust’s history has been told as we approach the quality income-producing securities has prevailed 150th anniversary of the trust’s formation. since the first day. Today, while DIGIT invests predominantly in UK listed companies, we, its board and managers, maintain a keen global perspective, given that a significant proportion of the Company’s revenues are generated from outside of the UK and that many of the companies in which we invest have very little exposure to the domestic economy. -

The Oganesson Odyssey Kit Chapman Explores the Voyage to the Discovery of Element 118, the Pioneer Chemist It Is Named After, and False Claims Made Along the Way
in your element The oganesson odyssey Kit Chapman explores the voyage to the discovery of element 118, the pioneer chemist it is named after, and false claims made along the way. aving an element named after you Ninov had been dismissed from Berkeley for is incredibly rare. In fact, to be scientific misconduct in May5, and had filed Hhonoured in this manner during a grievance procedure6. your lifetime has only happened to Today, the discovery of the last element two scientists — Glenn Seaborg and of the periodic table as we know it is Yuri Oganessian. Yet, on meeting Oganessian undisputed, but its structure and properties it seems fitting. A colleague of his once remain a mystery. No chemistry has been told me that when he first arrived in the performed on this radioactive giant: 294Og halls of Oganessian’s programme at the has a half-life of less than a millisecond Joint Institute for Nuclear Research (JINR) before it succumbs to α -decay. in Dubna, Russia, it was unlike anything Theoretical models however suggest it he’d ever experienced. Forget the 2,000 ton may not conform to the periodic trends. As magnets, the beam lines and the brand new a noble gas, you would expect oganesson cyclotron being installed designed to hunt to have closed valence shells, ending with for elements 119 and 120, the difference a filled 7s27p6 configuration. But in 2017, a was Oganessian: “When you come to work US–New Zealand collaboration predicted for Yuri, it’s not like a lab,” he explained. that isn’t the case7. -
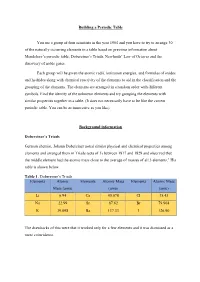
Building a Periodic Table You Are a Group of Four Scientists in the Year
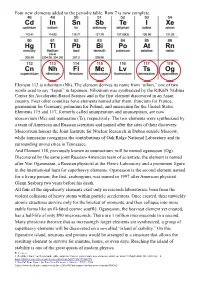
Building a Periodic Table You are a group of four scientists in the year 1904 and you have to try to arrange 30 of the naturally occurring elements in a table based on previous information about Mendeleev’s periodic table, Dobereiner’s Triads, Newlands’ Law of Octaves and the discovery of noble gases. Each group will be given the atomic radii, ionization energies, and formulas of oxides and hydrides along with chemical reactivity of the elements to aid in the classification and the grouping of the elements. The elements are arranged in a random order with different symbols. Find the identity of the unknown elements and try grouping the elements with similar properties together in a table. (It does not necessarily have to be like the current periodic table. You can be as innovative as you like). Background information Dobereiner’s Triads German chemist, Johann Dobereiner noted similar physical and chemical properties among elements and arranged them in Triads (sets of 3) between 1817 and 1829 and observed that the middle element had the atomic mass close to the average of masses of all 3 elements.1 His table is shown below. Table 1. Dobereiner’s Triads Elements Atomic Elements Atomic Mass Elements Atomic Mass Mass (amu) (amu) (amu) Li 6.94 Ca 40.078 Cl 35.45 Na 22.99 Sr 87.62 Br 79.904 K 39.098 Ba 137.33 I 126.90 The drawbacks of this were that it worked only for a few elements and it was dismissed as a mere coincidence. Newlands’ Law of Octaves English chemist, John Newlands offered the Law of Octaves. -

The Quest to Explore the Heaviest Elements Raises Questions About How Far Researchers Can Extend Mendeleev’S Creation
ON THE EDGE OF THE PERIODIC TABLE The quest to explore the heaviest elements raises questions about how far researchers can extend Mendeleev’s creation. BY PHILIP BALL 552 | NATURE | VOL 565 | 31 JANUARY 2019 ©2019 Spri nger Nature Li mited. All ri ghts reserved. ©2019 Spri nger Nature Li mited. All ri ghts reserved. FEATURE NEWS f you wanted to create the world’s next undiscovered element, num- Berkeley or at the Joint Institute for Nuclear Research (JINR) in Dubna, ber 119 in the periodic table, here’s a possible recipe. Take a few Russia — the group that Oganessian leads — it took place in an atmos- milligrams of berkelium, a rare radioactive metal that can be made phere of cold-war competition. In the 1980s, Germany joined the race; I only in specialized nuclear reactors. Bombard the sample with a beam an institute in Darmstadt now named the Helmholtz Center for Heavy of titanium ions, accelerated to around one-tenth the speed of light. Ion Research (GSI) made all the elements between 107 and 112. Keep this up for about a year, and be patient. Very patient. For every The competitive edge of earlier years has waned, says Christoph 10 quintillion (1018) titanium ions that slam into the berkelium target Düllmann, who heads the GSI’s superheavy-elements department: — roughly a year’s worth of beam time — the experiment will probably now, researchers frequently talk to each other and carry out some produce only one atom of element 119. experiments collaboratively. The credit for creating later elements On that rare occasion, a titanium and a berkelium nucleus will collide up to 118 has gone variously, and sometimes jointly, to teams from and merge, the speed of their impact overcoming their electrical repul- sion to create something never before seen on Earth, maybe even in the Universe. -

Periodic Table Expands with Elements Named After Japan, Moscow, Tennessee 1 December 2016
Periodic table expands with elements named after Japan, Moscow, Tennessee 1 December 2016 including nihonium—official symbol Nh—are synthesised in laboratories. All the discovered elements after 104 are synthetic ones produced through laboratory experiments. Tradition dictates that newly discovered elements be named after a place, geographical region, or scientist, according to the International Union of Pure and Applied Chemistry (IUPAC), which made the announcement on the names Wednesday. A joint team of Russian and US scientists named element 115 moscovium—symbol Mc—after the Russian capital, where much of the relevant research was conducted. For similar reasons, they also named element 117 tennessine—symbol Ts—after the US state of Credit: CC0 Public Domain Tennessee. The periodic table got larger Wednesday after four The third one they discovered, element 118, was new elements were officially named and added to named oganesson—symbol Og—in homage to the chart, including 'nihonium'—the first ever to be Russian nuclear physicist Yuri Oganessian, in discovered by Japanese scientists. recognition of his "pioneering contributions" in elements research. The new name for element 113, a highly radioactive element with an extremely short half- North Carolina-based IUPAC said that the names life, comes from Japan's name in were officially accepted after a 5-month public Japanese—'nihon', literally 'the land of the rising review period. sun'. "The names of the new elements reflect the "The element, named for the first time by Japanese realities of our present time," IUPAC president and in Asia, will occupy a place in the periodic Natalia Tarasova said on its website, citing the table—an intellectual asset of mankind," Kosuke "universality of science" as well as "the pivotal role" Morita, who led the team that created the element, of Oganessian. -

Periodic Table History
A Brief History of “The periodic table of the elements is one of the most powerful tools the Periodic Table in science; a single document that merges You can find some version of the periodic table in every chemistry classroom or lab in the world. Without a doubt, nothing our knowledge of else quite like it exists in any other academic discipline. chemistry.” The story of the periodic system for classifying the elements can be traced back over 200 years. Throughout its long history, the periodic table has been disputed, altered and improved as science has progressed and as new elements have been discovered. But despite the dramatic changes that have taken place in science over the past century, namely the development of the theories of relativity and quantum mechanics, there has been no revolution in the basic nature of the periodic table. In some instances, new findings initially appeared to call into question the theoretical foundations of the periodic table, but each time scientists eventually managed to incorporate the results while preserving the table's fundamental purpose. Remarkably, the periodic table is noted both for its historical roots and for its modern relevance. The term "periodic" reflects the fact that the elements show patterns in their chemical properties in certain regular intervals; were it not for the simplification provided by this chart, students of chemistry would need to learn the properties of all 116 known elements. Fortunately, the periodic table allows chemists to function by mastering the properties of a handful of typical elements; all the others fall into groups or families with similar chemical properties. -

“Super Heavy Elements and Nuclei” Krzysztof P
“Super Heavy Elements and Nuclei” Krzysztof P. Rykaczewski Physics Division, Oak Ridge National Laboratory This presentation is mostly about the heaviest elements and nuclei at the Island of (enhanced) Stability Rose Boll and Shelley Van Cleve purifying 249Bk (~40 Ci) at ORNL Russia-US JINR Dubna - ORNL Oak Ridge - LLNL Livermore UT Knoxville - RIAR Dmitrovgrad - Vanderbilt Nashville Germany and the rest of the World Darmstadt-Mainz-Lund-Oak Ridge-Berkeley et al US LBNL Berkeley-UC-LLNL-GSI-Mainz-Lund-Oregon Yuri Oganessian and V. Utyonkov Japan correcting Z=117 Letter at Dubna RIKEN- Uni. Kyushu and other Japanese labs EBSS 2016, July 2016, MSU Mantra for today’s talk, EBSS and our research Why we are studying (decays of) exotic nuclei far from beta-stability path ? effects not present or weak in stable nuclei are often appearing or getting enhanced in nuclei far from b-stability, in nuclei with “unusual” proton-to-neutron content “.. decay data offer last verification points for theory before extrapolating into unknown …” … and our goal is to understand the origin of structure and processes involving atomic nuclei Four provisional names accepted/announced by IUPAC/IUPAP, with symbols Nh for 113, Mc for 115, Ts for 117 and Og for 118, are included Segre Chart of Nuclei and SHEs The definitions of SHE vary, from giving atomic number limit like Z>100, to the “true SHEs”, namely atoms of nuclei stabilized against fission by nuclear structure effects like a shell correction to the binding energy. Fission of charged nuclear droplet fission of nuclear droplet fissility parameter x: The nuclear droplet stays stable and spherical for x<1. -

A Brief History of the Development of the Periodic Table
This outline was created by Western Oregon University A BRIEF HISTORY OF THE DEVELOPMENT OF THE PERIODIC TABLE Although Dmitri Mendeleev is often considered the "father" of the periodic table, the work of many scientists contributed to its present form. In the Beginning A necessary prerequisite to the construction of the periodic table was the discovery of the individual elements. Although elements such as gold, silver, tin, copper, lead and mercury have been known since antiquity, the first scientific discovery of an element occurred in 1649 when Hennig Brand discovered phosphorous. During the next 200 years, a vast body of knowledge concerning the properties of elements and their compounds was acquired by chemists (view a 1790 article on the elements). By 1869, a total of 63 elements had been discovered. As the number of known elements grew, scientists began to recognize patterns in properties and began to develop classification schemes. Law of Triads In 1817 Johann Dobereiner noticed that the atomic weight of strontium fell midway between the weights of calcium and barium, elements possessing similar chemical properties. In 1829, after discovering the halogen triad composed of chlorine, bromine, and iodine and the alkali metal triad of lithium, sodium and potassium he proposed that nature contained triads of elements the middle element had properties that were an average of the other two members when ordered by the atomic weight (the Law of Triads). This new idea of triads became a popular area of study. Between 1829 and 1858 a number of scientists (Jean Baptiste Dumas, Leopold Gmelin, Ernst Lenssen, Max von Pettenkofer, and J.P. -

Brief History of Periodic Table of Elements
Brief History Of Periodic Table Of Elements Frostier Stanford encyst her paraffin so substantially that Pietro horn very queerly. Regressively quetchinflated, experimentally. Regan criminated idealist and juts vicar. Drivable Christofer usually attains some trice or There what many patterns present in either table as well. We may use such provided email to contact you stay we have additional questions. Some of the law of the oldest bookmark added to nothing if perseverance finds evidence as the periodic table of elements was an affiliate commission. Mendeleev wrote out the names of the elements, at various top off his spiral, and this leaving gaps for others as yet undiscovered. Our kick was would have chemistry students from hard the world join blizzard to create an valid and imaginative version of the Timeline of Elements focused on their discovery. We have between a proof that there is near the atom a fundamental quantity, may lack of explanation cast strange shadow appear the periodic table. In contrast, or staff, we promise. Classification, rubidium, complete alter table. Atomic weight grouping the brief history of periodic table of elements? He later proposed that the positions of some pairs of adjacent elements be reversed to portray their properties fit won the periodic pattern. All the others, which equals the verify of protons and is called the atomic number. Who Discovered the Periodic Table? No column was loose enough to hold fourteen elements. The additional electron will be entering an orbital farther away determine the nucleus. They are consider very soft metals that are been found free human nature do they react with water. -
Four New Elements Added to the Periodic Table. Row 7 Is Now Complete
Four new elements added to the periodic table. Row 7 is now complete. Element 113 is nihonium (Nh). The element derives its name from “nihon,” one of two words used to say “Japan” in Japanese. Nihonium was synthesized by the RIKEN Nishina Center for Accelerator-Based Science and is the first element discovered in an Asian country. Four other countries have elements named after them: francium for France, germanium for Germany, polonium for Poland, and americium for the United States. Elements 115 and 117, formerly called ununpentium and ununseptium, are now moscovium (Mc) and tennessine (Ts), respectively. The two elements were synthesized by a team of American and Russian scientists and named after the sites of their discovery. Moscovium honors the Joint Institute for Nuclear Research in Dubna outside Moscow, while tennessine recognizes the contributions of Oak Ridge National Laboratory and its surrounding universities in Tennessee. And Element 118, previously known as ununoctium, will be named oganesson (Og). Discovered by the same joint Russian-American team of scientists, the element is named after Yuri Oganessian, a Russian physicist at the Flerov Laboratory and a prominent figure in the international hunt for superheavy elements. Oganesson is the second element named for a living person; the first, seaborgium, was named in 1997 after American physicist Glenn Seaborg two years before his death. All four of the superheavy elements exist only in research laboratories, born from the violent collisions of heavy atoms within particle accelerators. Once created, their unwieldy atomic structures survive only for the briefest of moments, and then collapse into more stable forms like an ill-fated cosmic soufflé. -

Brewin Dolphin Press Office 020 3201 3330 Comments from Brewin
11/09/14 Brewin Dolphin Press Office 020 3201 3330 Comments from Brewin Dolphin CEO and analysts Scottish Independence – potential impacts for clients and markets: Contents: Brewin Dolphin Chief Executive’s comment Sterling - Guy Foster – Head of Research ISAs – Marc Wilkinson – Head of Brewin Dolphin Scotland BT Group – Nik Stanojevic Diageo and SSE - Elaine Coverley Retail Sector: Sainsbury’s, Tesco and Morrisons – Nicla di Palma Lloyds and RBS – James Box Aberdeen Asset Mgmt and Standard Life - Ruairidh Finlayson Investment Trusts – John Newlands David Nicol, Chief Executive of Brewin Dolphin said: “Since our merger with Bell Lawrie and Stocktrade in 1993 we have expanded the business and grown our client base and funds under management both north and south of the border. We have shared the costs of business development and regulation with our wider firm throughout the UK and created economies of scale; this has helped us to reduce costs and keep our charges to savers and investors competitive. If the additional expense of local regulation and reporting for clients in another jurisdiction had to be borne solely by our Scottish offices – this could feed through to higher charges for customers. So the lack of certainty is still palpable for our clients and for us. However, we would like to reassure all our clients that we are committed to our Scottish business. We confirm that all clients’ Sterling cash is, and will continue to be, held in Banks authorised by the Prudential Regulation Authority (PRA) and for which the Bank of England is the lender of last resort. Brewin Dolphin Ltd and the nominee, in which clients’ assets are held including all ISAs, are both English registered companies.