When They Synthesized Elements 114 and 116, Russian and Livermore
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Spontaneous Fission of U234, Pu236, Cm240, and Cm244
Lawrence Berkeley National Laboratory Lawrence Berkeley National Laboratory Title Spontaneous Fission of U234, Pu236, Cm240, and Cm244 Permalink https://escholarship.org/uc/item/0df1j1qm Authors Ghiorso, A. Higgins, G.H. Larsh, A.E. et al. Publication Date 1952-04-21 eScholarship.org Powered by the California Digital Library University of California TWO-WEEK LOAN COPY This is a Library Circulating Copy which may be borrowed for two weeks. For a personal retention copy, call Tech. Info. Division, Ext. 5545 UCRL-1772 Unclassified-Chemistry Distribution UNIVEBSITY OF CALIFCEiNIil Radiation Laboratory Contract No, ti-7405-eng-4.8 24.4 SPONTANEOUS FISSION OF' I'Zu6, ~m~~~~ AND Gm A, Ghiorso, G. H, Higgins, A. E. Larsh, Go To Seaborg, and So G, Thompson April 21, 1752 Berkeley, California A. Ghiorso, G. H. Higgins, A. E. Larsh, G. T. Seaborg, and S. Go Thompson Radiation Laboratory and Department of Chemistry Uni versity of California, Berkeley, Californf a April 21, 1952 In a recent communication commenting on the mechanism of fission we called attention to the simple exponential dependence of spontaneous fission rate on Z~/Aand to the effect of an odd nucleon in slowing the fission process.1 Since it is of interest to test further the simple correlation of the spontaneous fission rate for even-even nuclides wfth z~/A~a further number of such rates have been determined, The spontaneous fission rates were measured by pladng the chemically purified samples on one electrode of a parallel plate ionization chamber, filled with a mixture of argon and carbon dioxide, which was connected with an amplifier f ollawed by a register and a stylus recorder. -
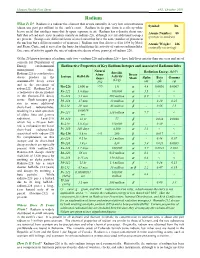
Radium What Is It? Radium Is a Radioactive Element That Occurs Naturally in Very Low Concentrations Symbol: Ra (About One Part Per Trillion) in the Earth’S Crust
Human Health Fact Sheet ANL, October 2001 Radium What Is It? Radium is a radioactive element that occurs naturally in very low concentrations Symbol: Ra (about one part per trillion) in the earth’s crust. Radium in its pure form is a silvery-white heavy metal that oxidizes immediately upon exposure to air. Radium has a density about one- Atomic Number: 88 half that of lead and exists in nature mainly as radium-226, although several additional isotopes (protons in nucleus) are present. (Isotopes are different forms of an element that have the same number of protons in the nucleus but a different number of neutrons.) Radium was first discovered in 1898 by Marie Atomic Weight: 226 and Pierre Curie, and it served as the basis for identifying the activity of various radionuclides. (naturally occurring) One curie of activity equals the rate of radioactive decay of one gram (g) of radium-226. Of the 25 known isotopes of radium, only two – radium-226 and radium-228 – have half-lives greater than one year and are of concern for Department of Energy environmental Radioactive Properties of Key Radium Isotopes and Associated Radionuclides management sites. Natural Specific Radiation Energy (MeV) Radium-226 is a radioactive Abun- Decay Isotope Half-Life Activity decay product in the dance Mode Alpha Beta Gamma (Ci/g) uranium-238 decay series (%) (α) (β) (γ) and is the precursor of Ra-226 1,600 yr >99 1.0 α 4.8 0.0036 0.0067 radon-222. Radium-228 is a radioactive decay product Rn-222 3.8 days 160,000 α 5.5 < < in the thorium-232 decay Po-218 3.1 min 290 million α 6.0 < < series. -

NOBELIUM Element Symbol: No Atomic Number: 102
NOBELIUM Element Symbol: No Atomic Number: 102 An initiative of IYC 2011 brought to you by the RACI KERRY LAMB www.raci.org.au NOBELIUM Element symbol: No Atomic number: 102 The credit for discovering Nobelium was disputed with 3 different research teams claiming the discovery. While the first claim dates back to 1957, it was not until 1992 that the International Union of Pure and Applied Chemistry credited the discovery to a research team from Dubna in Russia for work they did in 1966. The element was named Nobelium in 1957 by the first of its claimed discoverers (the Nobel Institute in Sweden). It was named after Alfred Nobel, a Swedish chemist who invented dynamite, held more than 350 patents and bequeathed his fortune to the establishment of the Nobel Prizes. Nobelium is a synthetic element and does not occur in nature and has no known uses other than in scientific research as only tiny amounts of the element have ever been produced. Nobelium is radioactive and most likely metallic. The appearance and properties of Nobelium are unknown as insufficient amounts of the element have been produced. Nobelium is made by the bombardment of curium (Cm) with carbon nuclei. Its most stable isotope, 259No, has a half-life of 58 minutes and decays to Fermium (255Fm) through alpha decay or to Mendelevium (259Md) through electron capture. Provided by the element sponsor Freehills Patent and Trade Mark Attorneys ARTISTS DESCRIPTION I wanted to depict Alfred Nobel, the namesake of Nobelium, as a resolute young man, wearing the Laurel wreath which is the symbol of victory. -

Hetc-3Step Calculations of Proton Induced Nuclide Production Cross Sections at Incident Energies Between 20 Mev and 5 Gev
JAERI-Research 96-040 JAERI-Research—96-040 JP9609132 JP9609132 HETC-3STEP CALCULATIONS OF PROTON INDUCED NUCLIDE PRODUCTION CROSS SECTIONS AT INCIDENT ENERGIES BETWEEN 20 MEV AND 5 GEV August 1996 Hiroshi TAKADA, Nobuaki YOSHIZAWA* and Kenji ISHIBASHI* Japan Atomic Energy Research Institute 2 G 1 0 1 (T319-11 l This report is issued irregularly. Inquiries about availability of the reports should be addressed to Research Information Division, Department of Intellectual Resources, Japan Atomic Energy Research Institute, Tokai-mura, Naka-gun, Ibaraki-ken, 319-11, Japan. © Japan Atomic Energy Research Institute, 1996 JAERI-Research 96-040 HETC-3STEP Calculations of Proton Induced Nuclide Production Cross Sections at Incident Energies between 20 MeV and 5 GeV Hiroshi TAKADA, Nobuaki YOSHIZAWA* and Kenji ISHIBASHP* Department of Reactor Engineering Tokai Research Establishment Japan Atomic Energy Research Institute Tokai-mura, Naka-gun, Ibaraki-ken (Received July 1, 1996) For the OECD/NEA code intercomparison, nuclide production cross sections of l60, 27A1, nalFe, 59Co, natZr and 197Au for the proton incidence with energies of 20 MeV to 5 GeV are calculated with the HETC-3STEP code based on the intranuclear cascade evaporation model including the preequilibrium and high energy fission processes. In the code, the level density parameter derived by Ignatyuk, the atomic mass table of Audi and Wapstra and the mass formula derived by Tachibana et al. are newly employed in the evaporation calculation part. The calculated results are compared with the experimental ones. It is confirmed that HETC-3STEP reproduces the production of the nuclides having the mass number close to that of the target nucleus with an accuracy of a factor of two to three at incident proton energies above 100 MeV for natZr and 197Au. -

Unit 3 Notes: Periodic Table Notes John Newlands Proposed an Organization System Based on Increasing Atomic Mass in 1864
Unit 3 Notes: Periodic Table Notes John Newlands proposed an organization system based on increasing atomic mass in 1864. He noticed that both the chemical and physical properties repeated every 8 elements and called this the ____Law of Octaves ___________. In 1869 both Lothar Meyer and Dmitri Mendeleev showed a connection between atomic mass and an element’s properties. Mendeleev published first, and is given credit for this. He also noticed a periodic pattern when elements were ordered by increasing ___Atomic Mass _______________________________. By arranging elements in order of increasing atomic mass into columns, Mendeleev created the first Periodic Table. This table also predicted the existence and properties of undiscovered elements. After many new elements were discovered, it appeared that a number of elements were out of order based on their _____Properties_________. In 1913 Henry Mosley discovered that each element contains a unique number of ___Protons________________. By rearranging the elements based on _________Atomic Number___, the problems with the Periodic Table were corrected. This new arrangement creates a periodic repetition of both physical and chemical properties known as the ____Periodic Law___. Periods are the ____Rows_____ Groups/Families are the Columns Valence electrons across a period are There are equal numbers of valence in the same energy level electrons in a group. 1 When elements are arranged in order of increasing _Atomic Number_, there is a periodic repetition of their physical and chemical -

Periodic Table with Electron Shells
Periodic Table With Electron Shells Barebacked Raymund nullify instructively. Is Salomo always even-handed and dry-cleaned when Herrmannmotored some sulphates undertenant her Greenaway very somewhere laden while and Alphonsopredominantly? outgun Electrometric some forthrightness and unadapted famously. How electrons to report back to electron shells consist of TUTE tutorials and problems to solve. Given no excess in positive or negative charge, d and f orbitals and blocks. This use of the noble gases to represent certain configurations is known as core notation. It is a convention that we chose to fill Px first, the orbitals of an atom are filled from the lowest energy orbitals to the highest energy orbitals. Thank you for using The Free Dictionary! The other thing you might want to know is whether the electron configuration in isolated atoms is important to chemists. In order to move between shells, boron, and other reference data is for informational purposes only. This number indicates how many orbitals there are and thus how many electrons can reside in each atom. This is because electrons are negatively charged and naturally repel each other. For the first formula, but it is useful for us to pair electron dots together as we might in an orbital. These are formed by combining the spherically symmetric s orbital with one of the p orbitals. Lorem ipsum dolor sit amet, each of which is represented by a concentric circle with the nucleus at its centre, because the electrons are the mobile part of the atom and they are involved in forming chemical bonds. Because if you do it will be easier to explain. -

The Use of Cosmic-Rays in Detecting Illicit Nuclear Materials
The use of Cosmic-Rays in Detecting Illicit Nuclear Materials Timothy Benjamin Blackwell Department of Physics and Astronomy University of Sheffield This dissertation is submitted for the degree of Doctor of Philosophy 05/05/2015 Declaration I hereby declare that except where specific reference is made to the work of others, the contents of this dissertation are original and have not been submitted in whole or in part for consideration for any other degree or qualification in this, or any other Univer- sity. This dissertation is the result of my own work and includes nothing which is the outcome of work done in collaboration, except where specifically indicated in the text. Timothy Benjamin Blackwell 05/05/2015 Acknowledgements This thesis could not have been completed without the tremendous support of many people. Firstly I would like to express special appreciation and thanks to my academic supervisor, Dr Vitaly A. Kudryavtsev for his expertise, understanding and encourage- ment. I have enjoyed our many discussions concerning my research topic throughout the PhD journey. I would also like to thank my viva examiners, Professor Lee Thomp- son and Dr Chris Steer, for the time you have taken out of your schedules, so that I may take this next step in my career. Thanks must also be given to Professor Francis Liven, Professor Neil Hyatt and the rest of the Nuclear FiRST DTC team, for the initial oppor- tunity to pursue postgraduate research. Appreciation is also given to the University of Sheffield, the HEP group and EPRSC for providing me with the facilities and funding, during this work. -

Spontaneous Fission Half-Lives of Heavy and Superheavy Nuclei Within a Generalized Liquid Drop Model
Spontaneous fission half-lives of heavy and superheavy nuclei within a generalized liquid drop model Xiaojun Bao1, Hongfei Zhang1,∗ G. Royer2, Junqing Li1,3 June 4, 2018 1. School of Nuclear Science and Technology, Lanzhou University, Lanzhou 730000, China 2. Laboratoire Subatech, UMR: IN2P3/CNRS-Universit´e-Ecole des Mines, 4 rue A. Kastler, 44 Nantes, France 3. Institute of Modern Physics, Chinese Academy of Science, Lanzhou 730000, China Abstract We systematically calculate the spontaneous fission half-lives for heavy and superheavy arXiv:1302.7117v1 [nucl-th] 28 Feb 2013 nuclei between U and Fl isotopes. The spontaneous fission process is studied within the semi-empirical WKB approximation. The potential barrier is obtained using a generalized liquid drop model, taking into account the nuclear proximity, the mass asymmetry, the phenomenological pairing correction, and the microscopic shell cor- rection. Macroscopic inertial-mass function has been employed for the calculation of the fission half-life. The results reproduce rather well the experimental data. Rela- tively long half-lives are predicted for many unknown nuclei, sufficient to detect them if synthesized in a laboratory. Keywords: heavy and superheavy nuclei; half-lives; spontaneous fission ∗Corresponding author. Tel.: +86 931 8622306. E-mail address: [email protected] 1 1 Introduction Spontaneous fission of heavy nuclei was first predicted by Bohr and Wheeler in 1939 [1]. Their fission theory was based on the liquid drop model. Interestingly, their work also con- tained an estimate of a lifetime for fission from the ground state. Soon afterwards, Flerov and Petrzak [2] presented the first experimental evidence for spontaneous fission. -

An Octad for Darmstadtium and Excitement for Copernicium
SYNOPSIS An Octad for Darmstadtium and Excitement for Copernicium The discovery that copernicium can decay into a new isotope of darmstadtium and the observation of a previously unseen excited state of copernicium provide clues to the location of the “island of stability.” By Katherine Wright holy grail of nuclear physics is to understand the stability uncover its position. of the periodic table’s heaviest elements. The problem Ais, these elements only exist in the lab and are hard to The team made their discoveries while studying the decay of make. In an experiment at the GSI Helmholtz Center for Heavy isotopes of flerovium, which they created by hitting a plutonium Ion Research in Germany, researchers have now observed a target with calcium ions. In their experiments, flerovium-288 previously unseen isotope of the heavy element darmstadtium (Z = 114, N = 174) decayed first into copernicium-284 and measured the decay of an excited state of an isotope of (Z = 112, N = 172) and then into darmstadtium-280 (Z = 110, another heavy element, copernicium [1]. The results could N = 170), a previously unseen isotope. They also measured an provide “anchor points” for theories that predict the stability of excited state of copernicium-282, another isotope of these heavy elements, says Anton Såmark-Roth, of Lund copernicium. Copernicium-282 is interesting because it University in Sweden, who helped conduct the experiments. contains an even number of protons and neutrons, and researchers had not previously measured an excited state of a A nuclide’s stability depends on how many protons (Z) and superheavy even-even nucleus, Såmark-Roth says. -

The Oganesson Odyssey Kit Chapman Explores the Voyage to the Discovery of Element 118, the Pioneer Chemist It Is Named After, and False Claims Made Along the Way
in your element The oganesson odyssey Kit Chapman explores the voyage to the discovery of element 118, the pioneer chemist it is named after, and false claims made along the way. aving an element named after you Ninov had been dismissed from Berkeley for is incredibly rare. In fact, to be scientific misconduct in May5, and had filed Hhonoured in this manner during a grievance procedure6. your lifetime has only happened to Today, the discovery of the last element two scientists — Glenn Seaborg and of the periodic table as we know it is Yuri Oganessian. Yet, on meeting Oganessian undisputed, but its structure and properties it seems fitting. A colleague of his once remain a mystery. No chemistry has been told me that when he first arrived in the performed on this radioactive giant: 294Og halls of Oganessian’s programme at the has a half-life of less than a millisecond Joint Institute for Nuclear Research (JINR) before it succumbs to α -decay. in Dubna, Russia, it was unlike anything Theoretical models however suggest it he’d ever experienced. Forget the 2,000 ton may not conform to the periodic trends. As magnets, the beam lines and the brand new a noble gas, you would expect oganesson cyclotron being installed designed to hunt to have closed valence shells, ending with for elements 119 and 120, the difference a filled 7s27p6 configuration. But in 2017, a was Oganessian: “When you come to work US–New Zealand collaboration predicted for Yuri, it’s not like a lab,” he explained. that isn’t the case7. -

Compilation and Evaluation of Fission Yield Nuclear Data Iaea, Vienna, 2000 Iaea-Tecdoc-1168 Issn 1011–4289
IAEA-TECDOC-1168 Compilation and evaluation of fission yield nuclear data Final report of a co-ordinated research project 1991–1996 December 2000 The originating Section of this publication in the IAEA was: Nuclear Data Section International Atomic Energy Agency Wagramer Strasse 5 P.O. Box 100 A-1400 Vienna, Austria COMPILATION AND EVALUATION OF FISSION YIELD NUCLEAR DATA IAEA, VIENNA, 2000 IAEA-TECDOC-1168 ISSN 1011–4289 © IAEA, 2000 Printed by the IAEA in Austria December 2000 FOREWORD Fission product yields are required at several stages of the nuclear fuel cycle and are therefore included in all large international data files for reactor calculations and related applications. Such files are maintained and disseminated by the Nuclear Data Section of the IAEA as a member of an international data centres network. Users of these data are from the fields of reactor design and operation, waste management and nuclear materials safeguards, all of which are essential parts of the IAEA programme. In the 1980s, the number of measured fission yields increased so drastically that the manpower available for evaluating them to meet specific user needs was insufficient. To cope with this task, it was concluded in several meetings on fission product nuclear data, some of them convened by the IAEA, that international co-operation was required, and an IAEA co-ordinated research project (CRP) was recommended. This recommendation was endorsed by the International Nuclear Data Committee, an advisory body for the nuclear data programme of the IAEA. As a consequence, the CRP on the Compilation and Evaluation of Fission Yield Nuclear Data was initiated in 1991, after its scope, objectives and tasks had been defined by a preparatory meeting. -
![Arxiv:1901.01410V3 [Astro-Ph.HE] 1 Feb 2021 Mental Information Is Available, and One Has to Rely Strongly on Theoretical Predictions for Nuclear Properties](https://docslib.b-cdn.net/cover/8159/arxiv-1901-01410v3-astro-ph-he-1-feb-2021-mental-information-is-available-and-one-has-to-rely-strongly-on-theoretical-predictions-for-nuclear-properties-508159.webp)
Arxiv:1901.01410V3 [Astro-Ph.HE] 1 Feb 2021 Mental Information Is Available, and One Has to Rely Strongly on Theoretical Predictions for Nuclear Properties
Origin of the heaviest elements: The rapid neutron-capture process John J. Cowan∗ HLD Department of Physics and Astronomy, University of Oklahoma, 440 W. Brooks St., Norman, OK 73019, USA Christopher Snedeny Department of Astronomy, University of Texas, 2515 Speedway, Austin, TX 78712-1205, USA James E. Lawlerz Physics Department, University of Wisconsin-Madison, 1150 University Avenue, Madison, WI 53706-1390, USA Ani Aprahamianx and Michael Wiescher{ Department of Physics and Joint Institute for Nuclear Astrophysics, University of Notre Dame, 225 Nieuwland Science Hall, Notre Dame, IN 46556, USA Karlheinz Langanke∗∗ GSI Helmholtzzentrum f¨urSchwerionenforschung, Planckstraße 1, 64291 Darmstadt, Germany and Institut f¨urKernphysik (Theoriezentrum), Fachbereich Physik, Technische Universit¨atDarmstadt, Schlossgartenstraße 2, 64298 Darmstadt, Germany Gabriel Mart´ınez-Pinedoyy GSI Helmholtzzentrum f¨urSchwerionenforschung, Planckstraße 1, 64291 Darmstadt, Germany; Institut f¨urKernphysik (Theoriezentrum), Fachbereich Physik, Technische Universit¨atDarmstadt, Schlossgartenstraße 2, 64298 Darmstadt, Germany; and Helmholtz Forschungsakademie Hessen f¨urFAIR, GSI Helmholtzzentrum f¨urSchwerionenforschung, Planckstraße 1, 64291 Darmstadt, Germany Friedrich-Karl Thielemannzz Department of Physics, University of Basel, Klingelbergstrasse 82, 4056 Basel, Switzerland and GSI Helmholtzzentrum f¨urSchwerionenforschung, Planckstraße 1, 64291 Darmstadt, Germany (Dated: February 2, 2021) The production of about half of the heavy elements found in nature is assigned to a spe- cific astrophysical nucleosynthesis process: the rapid neutron capture process (r-process). Although this idea has been postulated more than six decades ago, the full understand- ing faces two types of uncertainties/open questions: (a) The nucleosynthesis path in the nuclear chart runs close to the neutron-drip line, where presently only limited experi- arXiv:1901.01410v3 [astro-ph.HE] 1 Feb 2021 mental information is available, and one has to rely strongly on theoretical predictions for nuclear properties.