Antipsychotics
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Aripiprazole As an Adjunct to Anti-Depressants for Major Depressive Disorder: Clinical Effectiveness, Cost-Effectiveness, and Guidelines
TITLE: Aripiprazole as an Adjunct to Anti-Depressants for Major Depressive Disorder: Clinical Effectiveness, Cost-Effectiveness, and Guidelines DATE: 04 May 2016 RESEARCH QUESTIONS 1. What is the clinical efficacy of aripiprazole as an adjunct to antidepressants for the treatment of patients with major depressive disorder who have had an inadequate response to prior antidepressant treatments? 2. What is the cost-effectiveness of aripiprazole as an adjunct to antidepressants for the treatment of patients with major depressive disorder who have had an inadequate response to prior antidepressant treatments? 3. What are the evidence-based guidelines for the use of aripiprazole for the treatment of patients with major depressive disorder who have had an inadequate response to prior antidepressant treatments? KEY FINDINGS Three systematic reviews with meta-analyses, two randomized controlled trials, and two evidence-based guidelines were identified regarding the use of aripiprazole as an adjunct to antidepressants for the treatment of patients with major depressive disorder who have had an inadequate response to prior antidepressant treatments. METHODS A limited literature search was conducted on key resources including PubMed, Ovid PsychINFO, Ovid Embase, The Cochrane Library, University of York Centre for Reviews and Dissemination (CRD) databases, Canadian and major international health technology agencies, as well as a focused Internet search. No methodological filters were used to limit retrieval by study type. Where possible, retrieval was limited to the human population. The search was also limited to English language documents published between January 1, 2014 and April 29, 2016. Disclaimer: The Rapid Response Service is an information service for those involved in planning and providing health care in Canada. -

Original Research Published, Reproduced, Transmitted, Modified, Posted, Sold, Licensed, Or Used for Commercial Purposes
This work may not be copied, distributed, displayed, Original Research published, reproduced, transmitted, modified, posted, sold, licensed, or used for commercial purposes. By downloading this file, you are agreeing to the Efficacy and Effectiveness of Depot publisher’s Terms & Conditions. Versus Oral Antipsychotics in Schizophrenia: Synthesizing Results Across Different Research Designs Noam Y. Kirson, PhD; Peter J. Weiden, MD; Sander Yermakov, MS; Wayne Huang, MPP; Thomas Samuelson, BA; Steve J. Offord, PhD; Paul E. Greenberg, MS, MA; and Bruce J. O. Wong, MD ABSTRACT he cornerstone of long-term maintenance therapy Objective: Nonadherence is a major challenge in schizophrenia Tof schizophrenia patients is relapse prevention. treatment. While long-acting (depot) antipsychotic medications are Relapse prevention is necessary—albeit not sufficient— often recommended to address adherence problems, evidence on for eventual successful rehabilitation.1 In practice, the the comparative effectiveness of depot versus oral antipsychotics is effectiveness of maintenance antipsychotic treatment is inconsistent. We hypothesize that this inconsistency could be due to often undermined by poor adherence to therapy. Not systematic differences in study design. This review evaluates the effect only is nonadherence the single greatest modifiable of study design on the comparative effectiveness of antipsychotic risk factor for relapse,2,3 it is also often undetected, formulations. The optimal use of different antipsychotic formulations resulting in lost opportunities to employ psychosocial in a general clinical setting depends on better understanding of the underlying reasons for differences in effectiveness across research interventions for adherence, as well as uncertainty as designs. to the relative contribution of lack of efficacy versus Data Sources: A PubMed literature review targeted English-language adherence problems to poor outcomes. -

When Clozapine Is Not Tolerated Mitchell J
University of North Dakota UND Scholarly Commons Nursing Capstones Department of Nursing 10-28-2018 When Clozapine is Not Tolerated Mitchell J. Relf Follow this and additional works at: https://commons.und.edu/nurs-capstones Recommended Citation Relf, Mitchell J., "When Clozapine is Not Tolerated" (2018). Nursing Capstones. 268. https://commons.und.edu/nurs-capstones/268 This Independent Study is brought to you for free and open access by the Department of Nursing at UND Scholarly Commons. It has been accepted for inclusion in Nursing Capstones by an authorized administrator of UND Scholarly Commons. For more information, please contact [email protected]. Running head: WHEN CLOZAPINE IS NOT TOLERATED 1 WHEN CLOZAPINE IS NOT TOLERATED by Mitchell J. Relf Masters of Science in Nursing, University of North Dakota, 2018 An Independent Study Submitted to the Graduate Faculty of the University of North Dakota in partial fulfillment of the requirements for the degree of Master of Science Grand Forks, North Dakota December 2018 WHEN CLOZAPINE IS NOT TOLERATED 2 PERMISSION Title When Clozapine is Not Tolerated Department Nursing Degree Master of Science In presenting this independent study in partial fulfillment of the requirements for a graduate degree from the University of North Dakota, I agree that the College of Nursing and Professional Disciplines of this University shall make it freely available for inspection. I further agree that permission for extensive copying or electronic access for scholarly purposes may be granted by the professor who supervised my independent study work or, in her absence, by the chairperson of the department or the dean of the School of Graduate Studies. -

Treatment of Psychosis: 30 Years of Progress
Journal of Clinical Pharmacy and Therapeutics (2006) 31, 523–534 REVIEW ARTICLE Treatment of psychosis: 30 years of progress I. R. De Oliveira* MD PhD andM.F.Juruena MD *Department of Neuropsychiatry, School of Medicine, Federal University of Bahia, Salvador, BA, Brazil and Department of Psychological Medicine, Institute of Psychiatry, King’s College, University of London, London, UK phrenia. Thirty years ago, psychiatrists had few SUMMARY neuroleptics available to them. These were all Background: Thirty years ago, psychiatrists had compounds, today known as conventional anti- only a few choices of old neuroleptics available to psychotics, and all were liable to cause severe extra them, currently defined as conventional or typical pyramidal side-effects (EPS). Nowadays, new antipsychotics, as a result schizophrenics had to treatments are more ambitious, aiming not only to suffer the severe extra pyramidal side effects. improve psychotic symptoms, but also quality of Nowadays, new treatments are more ambitious, life and social reinsertion. We briefly but critically aiming not only to improve psychotic symptoms, outline the advances in diagnosis and treatment but also quality of life and social reinsertion. Our of schizophrenia, from the mid 1970s up to the objective is to briefly but critically review the present. advances in the treatment of schizophrenia with antipsychotics in the past 30 years. We conclude DIAGNOSIS OF SCHIZOPHRENIA that conventional antipsychotics still have a place when just the cost of treatment, a key factor in Up until the early 1970s, schizophrenia diagnoses poor regions, is considered. The atypical anti- remained debatable. The lack of uniform diagnostic psychotic drugs are a class of agents that have criteria led to relative rates of schizophrenia being become the most widely used to treat a variety of very different, for example, in New York and psychoses because of their superiority with London, as demonstrated in an important study regard to extra pyramidal symptoms. -

Download Executive Summary
Comparative Effectiveness Review Number 43 Effective Health Care Program Off-Label Use of Atypical Antipsychotics: An Update Executive Summary Background Effective Health Care Program Antipsychotics medications are approved The Effective Health Care Program by the U.S. Food and Drug Administration was initiated in 2005 to provide valid (FDA) for treatment of schizophrenia and evidence about the comparative bipolar disorder. These medications are effectiveness of different medical commonly divided into two classes, interventions. The object is to help reflecting two waves of historical consumers, health care providers, and development: the conventional others in making informed choices antipsychotics and the atypical. The among treatment alternatives. Through conventional antipsychotics served as the its Comparative Effectiveness Reviews, first successful pharmacologic treatment the program supports systematic for primary psychotic disorders such as appraisals of existing scientific schizophrenia. Having been widely used evidence regarding treatments for for decades, the conventional high-priority health conditions. It also antipsychotics also produced various side promotes and generates new scientific effects requiring additional medications, evidence by identifying gaps in which spurred the development of the existing scientific evidence and atypical antipsychotics. supporting new research. The program Currently, nine atypical antipsychotic drugs puts special emphasis on translating have been approved by FDA: aripiprazole, findings into a variety of useful asenapine, clozapine, iloperidone, formats for different stakeholders, olanzapine, paliperidone, quetiapine, including consumers. risperidone, and ziprasidone. These drugs The full report and this summary are have been used off-label (i.e., for available at www.effectivehealthcare. indications not approved by FDA) for the ahrq.gov/reports/final.cfm. treatment of various psychiatric conditions. -

Lurasidone (Latuda) Reference Number: CP.PMN.50 Effective Date: 09.01.15 Last Review Date: 02.21 Line of Business: Commercial, HIM, Medicaid Revision Log
Clinical Policy: Lurasidone (Latuda) Reference Number: CP.PMN.50 Effective Date: 09.01.15 Last Review Date: 02.21 Line of Business: Commercial, HIM, Medicaid Revision Log See Important Reminder at the end of this policy for important regulatory and legal information. Description Lurasidone (Latuda®) is an atypical antipsychotic. FDA Approved Indication(s) Latuda is indicated for the treatment of: • Schizophrenia in adults and adolescents (13 to 17 years) • Depressive episode associated with bipolar I disorder (bipolar depression) in adults and pediatric patients (10 to 17 years) as monotherapy • Depressive episode associated with bipolar I disorder (bipolar depression) in adults as adjunctive therapy with lithium or valproate Policy/Criteria Provider must submit documentation (such as office chart notes, lab results or other clinical information) supporting that member has met all approval criteria. It is the policy of health plans affiliated with Centene Corporation® that Latuda is medically necessary when the following criteria are met: I. Initial Approval Criteria A. Bipolar Disorder (must meet all): 1. Diagnosis of bipolar disorder; 2. Age ≥ 10 years; 3. Failure of two preferred atypical antipsychotics (e.g., aripiprazole, ziprasidone, quetiapine, risperidone, or olanzapine) at up to maximally indicated doses, each used for ≥ 4 weeks, unless all are contraindicated or clinically significant adverse effects are experienced; 4. Dose does not exceed 120 mg per day for adults and 80 mg per day for pediatric patients. Approval duration: Medicaid/HIM – 12 months Commercial – Length of Benefit B. Schizophrenia (must meet all): 1. Diagnosis of schizophrenia; 2. Age ≥ 13 years; 3. Failure of two preferred atypical antipsychotics (e.g., aripiprazole, ziprasidone, quetiapine, risperidone, or olanzapine) at up to maximally indicated doses, each used Page 1 of 6 CLINICAL POLICY Lurasidone for ≥ 4 weeks, unless all are contraindicated or clinically significant adverse effects are experienced; 4. -
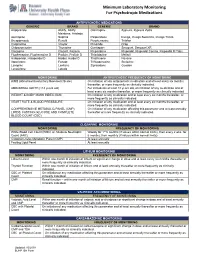
Minimum Laboratory Monitoring for Psychotropic Medications
Minimum Laboratory Monitoring For Psychotropic Medications ANTIPSYCHOTIC MEDICATIONS GENERIC BRAND GENERIC BRAND Aripiprazole Abilify, Abilify Olanzapine Zyprexa, Zyprexa Zydis Maintena, Aristada Asenapine Saphris Paliperidone Invega, Invega Sustenna, Invega Trinza Brexpiprazole Rexulti Perphenazine Trilafon Cariprazine Vraylar Pimozide Orap Chlorpromazine Thorazine Quetiapine Seroquel, Seroquel XR Clozapine Clozaril, Fazaclo Risperidone Risperdal, Risperdal Consta, Risperdal M Tabs Fluphenazine, Fluphenazine D Prolixin, Prolixin D Thioridazine Mellaril Haloperidol, Haloperidol D Haldol, Haldol D Thiothixene Navane Iloperidone Fanapt Trifluoperazine Stelazine Loxapine Loxitane Ziprasidone Geodon Lurasidone Latuda MONITORING ANTIPSYCHOTIC FREQUENCY OF MONITORING AIMS (Abnormal Involuntary Movement Scale) On initiation of any antipsychotic medication and at least every six months thereafter, or more frequently as clinically indicated. ABDOMINAL GIRTH (>18 years old) For individuals at least 18 years old, on initiation of any medication and at least every six months thereafter, or more frequently as clinically indicated. WEIGHT & BODY MASS INDEX (BMI) On initiation of any medication and at least every six months thereafter, or more frequently as clinically indicated. HEART RATE & BLOOD PRESSSURE On initiation of any medication and at least every six months thereafter, or more frequently as clinically indicated. COMPREHENSIVE METABOLIC PANEL (CMP), On initiation of any medication affecting this parameter and at least annually LIPIDS, FASTING -

Schizophrenia Care Guide
August 2015 CCHCS/DHCS Care Guide: Schizophrenia SUMMARY DECISION SUPPORT PATIENT EDUCATION/SELF MANAGEMENT GOALS ALERTS Minimize frequency and severity of psychotic episodes Suicidal ideation or gestures Encourage medication adherence Abnormal movements Manage medication side effects Delusions Monitor as clinically appropriate Neuroleptic Malignant Syndrome Danger to self or others DIAGNOSTIC CRITERIA/EVALUATION (PER DSM V) 1. Rule out delirium or other medical illnesses mimicking schizophrenia (see page 5), medications or drugs of abuse causing psychosis (see page 6), other mental illness causes of psychosis, e.g., Bipolar Mania or Depression, Major Depression, PTSD, borderline personality disorder (see page 4). Ideas in patients (even odd ideas) that we disagree with can be learned and are therefore not necessarily signs of schizophrenia. Schizophrenia is a world-wide phenomenon that can occur in cultures with widely differing ideas. 2. Diagnosis is made based on the following: (Criteria A and B must be met) A. Two of the following symptoms/signs must be present over much of at least one month (unless treated), with a significant impact on social or occupational functioning, over at least a 6-month period of time: Delusions, Hallucinations, Disorganized Speech, Negative symptoms (social withdrawal, poverty of thought, etc.), severely disorganized or catatonic behavior. B. At least one of the symptoms/signs should be Delusions, Hallucinations, or Disorganized Speech. TREATMENT OPTIONS MEDICATIONS Informed consent for psychotropic -

Management of Side Effects of Antipsychotics
Management of side effects of antipsychotics Oliver Freudenreich, MD, FACLP Co-Director, MGH Schizophrenia Program www.mghcme.org Disclosures I have the following relevant financial relationship with a commercial interest to disclose (recipient SELF; content SCHIZOPHRENIA): • Alkermes – Consultant honoraria (Advisory Board) • Avanir – Research grant (to institution) • Janssen – Research grant (to institution), consultant honoraria (Advisory Board) • Neurocrine – Consultant honoraria (Advisory Board) • Novartis – Consultant honoraria • Otsuka – Research grant (to institution) • Roche – Consultant honoraria • Saladax – Research grant (to institution) • Elsevier – Honoraria (medical editing) • Global Medical Education – Honoraria (CME speaker and content developer) • Medscape – Honoraria (CME speaker) • Wolters-Kluwer – Royalties (content developer) • UpToDate – Royalties, honoraria (content developer and editor) • American Psychiatric Association – Consultant honoraria (SMI Adviser) www.mghcme.org Outline • Antipsychotic side effect summary • Critical side effect management – NMS – Cardiac side effects – Gastrointestinal side effects – Clozapine black box warnings • Routine side effect management – Metabolic side effects – Motor side effects – Prolactin elevation • The man-in-the-arena algorithm www.mghcme.org Receptor profile and side effects • Alpha-1 – Hypotension: slow titration • Dopamine-2 – Dystonia: prophylactic anticholinergic – Akathisia, parkinsonism, tardive dyskinesia – Hyperprolactinemia • Histamine-1 – Sedation – Weight gain -

Revision of Precautions Asenapine Maleate, Aripiprazole, Olanzapine
Published by Translated by Ministry of Health, Labour and Welfare Pharmaceuticals and Medical Devices Agency This English version is intended to be a reference material to provide convenience for users. In the event of inconsistency between the Japanese original and this English translation, the former shall prevail. Revision of Precautions Asenapine maleate, aripiprazole, olanzapine, quetiapine fumarate, clocapramine hydrochloride hydrate, chlorpromazine hydrochloride, chlorpromazine hydrochloride/promethazine hydrochloride/phenobarbital, chlorpromazine phenolphthalinate, spiperone, zotepine, timiperone, haloperidol, paliperidone, pipamperone hydrochloride, fluphenazine decanoate, fluphenazine maleate, brexpiprazole, prochlorperazine maleate, prochlorperazine mesilate, Pharmaceuticals and Medical Devices Agency Office of Safety I 3-3-2 Kasumigaseki, Chiyoda-ku, Tokyo 100-0013 Japan E-mail: [email protected] Published by Translated by Ministry of Health, Labour and Welfare Pharmaceuticals and Medical Devices Agency This English version is intended to be a reference material to provide convenience for users. In the event of inconsistency between the Japanese original and this English translation, the former shall prevail. propericiazine, bromperidol, perphenazine, perphenazine hydrochloride, perphenazine fendizoate, perphenazine maleate, perospirone hydrochloride hydrate, mosapramine hydrochloride, risperidone (oral drug), levomepromazine hydrochloride, levomepromazine maleate March 27, 2018 Non-proprietary name Asenapine maleate, -

Current P SYCHIATRY
Current p SYCHIATRY N ew Investigators Tips to manage and prevent discontinuation syndromes Informed tapering can protect patients when you stop a medication Sriram Ramaswamy, MD Shruti Malik, MBBS, MHSA Vijay Dewan, MD Instructor, department of psychiatry Foreign medical graduate Assistant professor Creighton University Department of psychiatry Omaha, NE University of Nebraska Medical Center Omaha, NE bruptly stopping common psychotropics New insights on psychotropic A —particularly antidepressants, benzodi- drug safety and side effects azepines, or atypical antipsychotics—can trigger a discontinuation syndrome, with: This paper was among those entered in the 2005 • rebound or relapse of original symptoms Promising New Investigators competition sponsored • uncomfortable new physical and psycho- by the Neuroleptic Malignant Syndrome Information Service (NMSIS). The theme of this year’s competition logical symptoms was “New insights on psychotropic drug safety and • physiologic withdrawal at times. side effects.” To increase health professionals’ awareness of URRENT SYCHIATRY 1 C P is honored to publish this peer- the risk of these adverse effects, this article reviewed, evidence-based article on a clinically describes discontinuation syndromes associated important topic for practicing psychiatrists. with various psychotropics and offers strategies to NMSIS is dedicated to reducing morbidity and anticipate, recognize, and manage them. mortality of NMS by improving medical and psychiatric care of patients with heat-related disorders; providing -

Drug Repurposing for the Management of Depression: Where Do We Stand Currently?
life Review Drug Repurposing for the Management of Depression: Where Do We Stand Currently? Hosna Mohammad Sadeghi 1,†, Ida Adeli 1,† , Taraneh Mousavi 1,2, Marzieh Daniali 1,2, Shekoufeh Nikfar 3,4,5 and Mohammad Abdollahi 1,2,* 1 Toxicology and Diseases Group (TDG), Pharmaceutical Sciences Research Center (PSRC), The Institute of Pharmaceutical Sciences (TIPS), Tehran University of Medical Sciences, Tehran 1417614411, Iran; [email protected] (H.M.S.); [email protected] (I.A.); [email protected] (T.M.); [email protected] (M.D.) 2 Department of Toxicology and Pharmacology, School of Pharmacy, Tehran University of Medical Sciences, Tehran 1417614411, Iran 3 Personalized Medicine Research Center, Endocrinology and Metabolism Research Institute, Tehran University of Medical Sciences, Tehran 1417614411, Iran; [email protected] 4 Pharmaceutical Sciences Research Center (PSRC) and the Pharmaceutical Management and Economics Research Center (PMERC), Evidence-Based Evaluation of Cost-Effectiveness and Clinical Outcomes Group, The Institute of Pharmaceutical Sciences (TIPS), Tehran University of Medical Sciences, Tehran 1417614411, Iran 5 Department of Pharmacoeconomics and Pharmaceutical Administration, School of Pharmacy, Tehran University of Medical Sciences, Tehran 1417614411, Iran * Correspondence: [email protected] † Equally contributed as first authors. Citation: Mohammad Sadeghi, H.; Abstract: A slow rate of new drug discovery and higher costs of new drug development attracted Adeli, I.; Mousavi, T.; Daniali, M.; the attention of scientists and physicians for the repurposing and repositioning of old medications. Nikfar, S.; Abdollahi, M. Drug Experimental studies and off-label use of drugs have helped drive data for further studies of ap- Repurposing for the Management of proving these medications.