Original Research Published, Reproduced, Transmitted, Modified, Posted, Sold, Licensed, Or Used for Commercial Purposes
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Revision of Precautions Asenapine Maleate, Aripiprazole, Olanzapine
Published by Translated by Ministry of Health, Labour and Welfare Pharmaceuticals and Medical Devices Agency This English version is intended to be a reference material to provide convenience for users. In the event of inconsistency between the Japanese original and this English translation, the former shall prevail. Revision of Precautions Asenapine maleate, aripiprazole, olanzapine, quetiapine fumarate, clocapramine hydrochloride hydrate, chlorpromazine hydrochloride, chlorpromazine hydrochloride/promethazine hydrochloride/phenobarbital, chlorpromazine phenolphthalinate, spiperone, zotepine, timiperone, haloperidol, paliperidone, pipamperone hydrochloride, fluphenazine decanoate, fluphenazine maleate, brexpiprazole, prochlorperazine maleate, prochlorperazine mesilate, Pharmaceuticals and Medical Devices Agency Office of Safety I 3-3-2 Kasumigaseki, Chiyoda-ku, Tokyo 100-0013 Japan E-mail: [email protected] Published by Translated by Ministry of Health, Labour and Welfare Pharmaceuticals and Medical Devices Agency This English version is intended to be a reference material to provide convenience for users. In the event of inconsistency between the Japanese original and this English translation, the former shall prevail. propericiazine, bromperidol, perphenazine, perphenazine hydrochloride, perphenazine fendizoate, perphenazine maleate, perospirone hydrochloride hydrate, mosapramine hydrochloride, risperidone (oral drug), levomepromazine hydrochloride, levomepromazine maleate March 27, 2018 Non-proprietary name Asenapine maleate, -

Is Aristada (Aripiprazole Lauroxil) a Safe and Effective Treatment for Schizophrenia in Adult Patients? Kyle J
Philadelphia College of Osteopathic Medicine DigitalCommons@PCOM PCOM Physician Assistant Studies Student Student Dissertations, Theses and Papers Scholarship 2017 Is Aristada (Aripiprazole Lauroxil) a Safe and Effective Treatment For Schizophrenia In Adult Patients? Kyle J. Knowles Philadelphia College of Osteopathic Medicine Follow this and additional works at: https://digitalcommons.pcom.edu/pa_systematic_reviews Part of the Psychiatry Commons Recommended Citation Knowles, Kyle J., "Is Aristada (Aripiprazole Lauroxil) a Safe and Effective Treatment For Schizophrenia In Adult Patients?" (2017). PCOM Physician Assistant Studies Student Scholarship. 381. https://digitalcommons.pcom.edu/pa_systematic_reviews/381 This Selective Evidence-Based Medicine Review is brought to you for free and open access by the Student Dissertations, Theses and Papers at DigitalCommons@PCOM. It has been accepted for inclusion in PCOM Physician Assistant Studies Student Scholarship by an authorized administrator of DigitalCommons@PCOM. For more information, please contact [email protected]. Is Aristada (Aripiprazole Lauroxil) a Safe and Effective Treatment For Schizophrenia In Adult Patients? Kyle J. Knowles, PA-S A SELECTIVE EVIDENCE BASED MEDICINE REVIEW In Partial Fulfillment of the Requirements For The Degree of Master of Science In Health Sciences- Physician Assistant Department of Physician Assistant Studies Philadelphia College of Osteopathic Medicine Philadelphia, Pennsylvania December 16, 2016 ABSTRACT OBJECTIVE: The objective of this selective EBM review is to determine whether or not “Is Aristada (aripiprazole lauroxil) a safe and effective treatment for schizophrenia in adult patients?” STUDY DESIGN: Review of three randomized controlled studies. All three trials were conducted between 2014 and 2015. DATA SOURCES: One randomized, controlled trial and two randomized, controlled, double- blind trials found via Cochrane Library and PubMed. -

A Brief Overview of Psychiatric Pharmacotherapy
A Brief Overview of Psychiatric Pharmacotherapy Joel V. Oberstar, M.D. Chief Executive Officer Disclosures • Some medications discussed are not approved by the FDA for use in the population discussed/described. • Some medications discussed are not approved by the FDA for use in the manner discussed/described. • Co-Owner: – PrairieCare and PrairieCare Medical Group – Catch LLC Disclaimer The contents of this handout are for informational purposes only and are not intended to be a substitute for professional medical advice, diagnosis, or treatment. Always seek the advice of your physician or other qualified healthcare provider with any questions you may have regarding a medical or psychiatric condition. Never disregard professional/medical advice or delay in seeking it because of something you have read in this handout. Material in this handout may be copyrighted by the author or by third parties; reasonable efforts have been made to give attribution where appropriate. Caveat Regarding the Role of Medication… Neuroscience Overview Mind Over Matter, National Institute on Drug Abuse, National Institutes of Health. Available at: http://teens.drugabuse.gov/mom/index.asp. http://medicineworld.org/images/news-blogs/brain-700997.jpg Neuroscience Overview Mind Over Matter, National Institute on Drug Abuse, National Institutes of Health. Available at: http://teens.drugabuse.gov/mom/index.asp. Neurotransmitter Receptor Source: National Institute on Drug Abuse Common Diagnoses and Associated Medications • Psychotic Disorders – Antipsychotics • Bipolar Disorders – Mood Stabilizers, Antipsychotics, & Antidepressants • Depressive Disorders – Antidepressants • Anxiety Disorders – Antidepressants & Anxiolytics • Attention Deficit Hyperactivity Disorder – Stimulants, Antidepressants, 2-Adrenergic Agents, & Strattera Classes of Medications • Anti-depressants • Stimulants and non-stimulant alternatives • Anti-psychotics (a.k.a. -

Psychotropic Medication Concerns When Treating Individuals with Developmental Disabilities
Tuesday, 2:30 – 4:00, C2 Psychotropic Medication Concerns when Treating Individuals with Developmental Disabilities Richard Berchou 248-613-6716 [email protected] Objective: Identify effective methods for the practical application of concepts related to improving the delivery of services for persons with developmental disabilities Notes: Medication Assistance On-Line Resources OBTAINING MEDICATION: • Needy Meds o Needymeds.com • Partnership for Prescriptions Assistance o Pparx.org • Patient Assistance Program Center o Rxassist.org • Insurance coverage & Prior authorization forms for most drug plans o Covermymeds.com REMINDERS TO TAKE MEDICATION: • Medication reminder by Email, Phone call, or Text message o Sugaredspoon.com ANSWER MOST QUESTIONS ABOUT MEDICATIONS: • Univ. of Michigan/West Virginia Schools of Pharmacy o Justaskblue.com • Interactions between medications, over-the-counter (OTC) products and some foods; also has a pictorial Pill Identifier: May input an entire list of medications o Drugs.com OTHER TRUSTED SITES: • Patient friendly information about disease and diagnoses o Mayoclinic.com, familydoctor.org • Package inserts, boxed warnings, “Dear Doctor” letters (can sign up to receive e- mail alerts) o Dailymed.nlm.nih.gov • Communications about drug safety o www.Fda.gov/cder/drug/drugsafety/drugindex.htm • Purchasing medications on-line o Pharnacychecker.com Updated 2013 Psychotropic Medication for Persons with Developmental Disabilities April 23, 2013 Richard Berchou, Pharm. D. Assoc. Clinical Prof., Dept. Psychiatry & Behavioral -

Antipsychotics
bmchp.org | 888-566-0008 wellsense.org | 877-957-1300 Pharmacy Policy Antipsychotics Policy Number: 9.503 Version Number: 2.1 Version Effective Date: 9/1/2021 Product Applicability All Plan+ Products Well Sense Health Plan Boston Medical Center HealthNet Plan New Hampshire Medicaid MassHealth - MCO MassHealth - ACO Qualified Health Plans/ConnectorCare/Employer Choice Direct Senior Care Options __________________________________ Note: Disclaimer and audit information is located at the end of this document. Prior Authorization Policy Products Affected: aripiprazole ODT Latuda (lurasidone) aripiprazole Sol Nuplazid (pimavanserin) asenapine maleate Rexulti (brexpiprazole) clozapine ODT Vraylar (cariprazine) risperidone ODT Versacloz (clozapine) Fanapt (iloperidone) Secuado (asenapine) Paliperidone The Plan may authorize coverage of the above products for members meeting the following criteria: Covered All FDA approved indications not otherwise excluded Use All indications supported by established clinical literature for the medical condition and age + Plan refers to Boston Medical Center Health Plan, Inc. and its affiliates and subsidiaries offering health coverage plans to enrolled members. The Plan operates in Massachusetts under the trade name Boston Medical Center HealthNet Plan and in other states under the trade name Well Sense Health Plan. Antipsychotics 1 of 6 Exclusion None Criteria Required Medical Information clozapine ODT, risperidone ODT, aripiprazole ODT, aripiprazole sol, Versacloz 1. A diagnosis of bipolar disorder, schizophrenia, or other psychotic disorder; OR 2. A diagnosis of major depression requiring adjunct therapy ( aripiprazole ODT, aripiprazole sol only); AND 3. Clinical swallowing difficulties (including unwillingness to swallow tablets) Fanapt, palipedione (oral), Latuda, Vraylar 1. A diagnosis of bipolar disorder, schizophrenia, or other psychotic disorder; AND 2. An inadequate response, intolerance or contraindication to a trial of two covered atypical antipsychotics (See Appendix A) Nuplazid 1. -
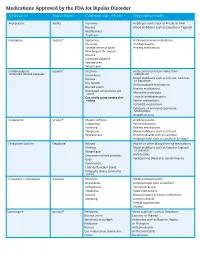
Medications Approved by the FDA for Bipolar Disorder Medication Brand Name Common Side Effects May Interact With
Medications Approved by the FDA for Bipolar Disorder Medication Brand Name Common side effects May interact with Aripiprazole Abilify® Insomnia Antidepressants such as Prozac or Paxil Nausea Mood stabilizers such as Equetro or Tegretol Restlessness Tiredness Asenapine Saphris® Sleepiness Antihypertensive medications Dizziness Antidepressants Strange sense of taste Anxiety medications Numbing of the mouth Nausea Increased appetite Feeling tired Weight gain Carbamazepine Equetro™ Dizziness Birth control pills (can make them extended release capsules Drowsiness ineffective) Mood stabilizers such as Lithium, Lamictal, Nausea or Depakote Dry mouth Anticonvulsant medications Blurred vision Anxiety medications Decreased white blood cell count Macrolide antibiotics Can rarely cause severe skin Tricyclic antidepressants rashes Cancer medications HIV/AIDS medications Cytotoxic or immunosuppressive Medications Grapefruit juice Cariprazine Vraylar® Muscle stiffness Antidepressants Indigestion Pain medications Vomiting Anxiety medications Sleepiness Mood stabilizers such as Lithium Restlessness Anticonvulsants such as Lamictal Antipsychotics such as Latuda or Seroquel Divalproex Sodium Depakote® Nausea Aspirin or other blood thinning medications Shaking Mood stabilizers such as Equetro,Tegretol, Weight gain or Lamictal Decrease in blood platelets Barbiturates Rash Cyclosporine (Neoral or Sandimmune) Pancreatitis Liver dysfunction (rare) Polycystic Ovary Syndrome (rare) Fluoxetine + Olanzapine Symbyax® Dizziness MAOI antidepressants Drowsiness Antipsychotics -

1 Louisiana Medicaid Behavioral Health Medications for Children
Louisiana Medicaid Behavioral Health Medications for Children Under 6 Years of Age For Medications Not Addressed on the Preferred/Non-Preferred Drug List The Louisiana Uniform Prescription Drug Prior Authorization Form should be utilized to request clinical authorization for all behavioral health medications for children under 6 years of age. Additional Point-of-Sale edits may apply. In addition to the therapeutic classes on the Preferred/Non-Preferred Drug List (PDL/NPDL) that include criteria for use in children under 6 years of age (Stimulants and Related Agents, Antidepressants, Antipsychotics, and Anxiolytics), the following behavioral health medications that are not found on the PDL/NPDL, when prescribed for a behavioral health diagnosis for children under 6 years of age, require authorization: Additional Antidepressants – Amitriptyline, Amitriptyline/Chlordiazepoxide, Amoxapine, Chlorpromazine, Clomipramine , Desipramine, Doxepin, Imipramine, Maprotiline, Nortriptyline, Protriptyline, Trimipramine Additional Antipsychotics – Asenapine Transdermal (Secuado®) Additional Anxiolytic Agents – Chlordiazepoxide/Clidinium, Clonazepam, Lorazepam Injection Mood Stabilizers – Carbamazepine (Equetro®), Lithium All of these agents have Black Box Warnings; see individual prescribing information for details. When a pharmacy claim for carbamazepine (Equetro®) or clonazepam for a child under 6 years of age is submitted with a diagnosis code for seizures, the claim will bypass the behavioral health authorization requirement. Initial and Reauthorization -
Psychotropic Drug Indications
PSYCHOTROPIC DRUG INDICATIONS (Part 1 of 2) Bipolar Disorder Mixed Generic Brand Form Mania Depression Episodes Maintenance MDD TRD PMDD Schizophrenia Others4 ATYPICAL ANTIPSYCHOTICS aripiprazole — tabs, ODT, oral √ √ √ √1 √ √ soln Abilify tabs √ √ √ √1 √ √ Abilify ext-rel IM inj √ √ Maintena Abilify tabs with √ √ √ √1 √ Mycite sensor aripiprazole Aristada ext-rel IM inj √ lauroxil Aristada ext-rel IM inj √ Initio asenapine Saphris sublingual tabs √ √ √ √ brexpiprazole Rexulti tabs √1 √ cariprazine Vraylar caps √ √ √ √ lurasidone Latuda tabs √ √ olanzapine Zyprexa tabs √ √2 √ √ √2 √ IM inj √ Zyprexa ext-rel IM inj √ Relprevv Zyprexa ODT √ √2 √ √ √2 √ Zydis quetiapine Seroquel tabs √ √ √ √ Seroquel XR ext-rel tabs √ √ √ √ √1 √ risperidone Perseris ext-rel SC inj √ Risperdal tabs, oral soln √ √ √ √ Risperdal ext-rel IM inj √ √ Consta Risperdal ODT √ √ √ √ M-tabs ziprasidone Geodon caps √ √ √1 √ IM inj √ COMBINATION ATYPICAL & SELECTIVE SEROTONIN REUPTAKE INHIBITOR olanzapine + Symbyax caps √ √ fluoxetine MONOAMINE OXIDASE INHIBITORS (MAOIs) phenelzine Nardil tabs √ selegiline EMSAM transdermal √ system tranylcypromine Parnate tabs √ SEROTONIN AND NOREPINEPHRINE REUPTAKE INHIBITORS (SNRIs) desvenlafaxine Khedezla ext-rel tabs √ Pristiq ext-rel tabs √ duloxetine Cymbalta caps √ √ levomilnacipran Fetzima ext-rel caps √ venlafaxine — scored tabs √ Effexor XR ext-rel caps √ √ SELECTIVE SEROTONIN REUPTAKE INHIBITORS (SSRIs) citalopram Celexa scored tabs √ escitalopram Lexapro scored tabs, √ √ oral soln fluoxetine — tabs, oral soln √3 √ √3 √ Prozac -

Medications to Be Avoided Or Used with Caution in Parkinson's Disease
Medications To Be Avoided Or Used With Caution in Parkinson’s Disease This medication list is not intended to be complete and additional brand names may be found for each medication. Every patient is different and you may need to take one of these medications despite caution against it. Please discuss your particular situation with your physician and do not stop any medication that you are currently taking without first seeking advice from your physician. Most medications should be tapered off and not stopped suddenly. Although you may not be taking these medications at home, one of these medications may be introduced while hospitalized. If a hospitalization is planned, please have your neurologist contact your treating physician in the hospital to advise which medications should be avoided. Medications to be avoided or used with caution in combination with Selegiline HCL (Eldepryl®, Deprenyl®, Zelapar®), Rasagiline (Azilect®) and Safinamide (Xadago®) Medication Type Medication Name Brand Name Narcotics/Analgesics Meperidine Demerol® Tramadol Ultram® Methadone Dolophine® Propoxyphene Darvon® Antidepressants St. John’s Wort Several Brands Muscle Relaxants Cyclobenzaprine Flexeril® Cough Suppressants Dextromethorphan Robitussin® products, other brands — found as an ingredient in various cough and cold medications Decongestants/Stimulants Pseudoephedrine Sudafed® products, other Phenylephrine brands — found as an ingredient Ephedrine in various cold and allergy medications Other medications Linezolid (antibiotic) Zyvox® that inhibit Monoamine oxidase Phenelzine Nardil® Tranylcypromine Parnate® Isocarboxazid Marplan® Note: Additional medications are cautioned against in people taking Monoamine oxidase inhibitors (MAOI), including other opioids (beyond what is mentioned in the chart above), most classes of antidepressants and other stimulants (beyond what is mentioned in the chart above). -

Application of a Pharmacogenetics-Based Precision Medicine Model (5SPM) to Psychotic Patients That Presented Poor Response to Neuroleptic Therapy
Journal of Personalized Medicine Article Application of a Pharmacogenetics-Based Precision Medicine Model (5SPM) to Psychotic Patients That Presented Poor Response to Neuroleptic Therapy Lorena Carrascal-Laso 1 , Manuel Ángel Franco-Martín 1,* , María Belén García-Berrocal 2 , Elena Marcos-Vadillo 2, Santiago Sánchez-Iglesias 3, Carolina Lorenzo 3, Almudena Sánchez-Martín 4, Ignacio Ramos-Gallego 5, M Jesús García-Salgado 2 and María Isidoro-García 2,6 1 Servicio de Psiquiatría, Hospital Provincial de Zamora, IBSAL, 49071 Zamora, Spain; [email protected] 2 Farmacogenética y Medicina de Precisión, Servicio de Bioquímica, Hospital Universitario de Salamanca, IBSAL, 37007 Salamanca, Spain; [email protected] (M.B.G.-B.); [email protected] (E.M.-V.); [email protected] (M.J.G.-S.); [email protected] (M.I.-G.) 3 Servicio de Psiquiatría, Hospital Universitario de Salamanca, IBSAL, 37007 Salamanca, Spain; [email protected] (S.S.-I.); [email protected] (C.L.) 4 Pharmacogenetics Unit, Pharmacy Department, University Hospital Virgen de las Nieves, UGC Provincial de Farmacia de Granada, Avda, Fuerzas Armadas, 18014 Granada, Spain; [email protected] 5 Departamento de Fisiología y Farmacología, Universidad de Salamanca, 37007 Salamanca, Spain; [email protected] 6 Departamento de Medicina, Universidad de Salamanca, 37007 Salamanca, Spain * Correspondence: [email protected] Received: 4 November 2020; Accepted: 15 December 2020; Published: 18 December 2020 Abstract: Antipsychotics are the keystone of the treatment of severe and prolonged mental disorders. However, there are many risks associated with these drugs and not all patients undergo full therapeutic profit from them. The application of the 5 Step Precision Medicine model(5SPM), based on the analysis of the pharmacogenetic profile of each patient, could be a helpful tool to solve many of the problematics traditionally associated with the neuroleptic treatment. -

Currently Prescribed Psychotropic Medications
CURRENTLY PRESCRIBED PSYCHOTROPIC MEDICATIONS Schizophrenia Depression Anxiety Disorders 1st generation antipsychotics: Tricyclics: Atarax (hydroxyzine) Haldol (haloperidol), *Anafranil (clomipramine) Ativan (lorazepam) Haldol Decanoate Asendin (amoxapine) BuSpar (buspirone) Loxitane (loxapine) Elavil (amitriptyline) *Inderal (propranolol) Mellaril (thioridazine) Norpramin (desipramine) Keppra (levetiracetam) Navane (thiothixene) Pamelor (nortriptyline) *Klonopin (clonazepam) Prolixin (fluphenazine), Prolixin Sinequan (doxepin) Librium (chlordiazepoxide) Decanoate Spravato (esketamine) Serax (oxazepam) Stelazine (trifluoperazine) Surmontil (trimipramine) Thorazine (chlorpromazine) *Tenormin (atenolol) Tofranil (imipramine) MEDICATIONS PSYCHOTROPIC PRESCRIBED CURRENTLY Trilafon (perphenazine) Tranxene (clorazepate) Vivactil (protriptyline) Valium (diazepam) 2nd generation antipsychotics: Zulresso (brexanolone) Vistaril (hydroxyzine) Abilify (aripiprazole) Aristada (aripiprazole) SSRIs: Xanax (alprazolam) Caplyta (lumateperone) Celexa (citalopram) *Antidepressants, especially SSRIs, are also used in the treatment of anxiety. Clozaril (clozapine) Lexapro (escitalopram) Fanapt (iloperidone) *Luvox (fluvoxamine) Geodon (ziprasidone) Paxil (paroxetine) Stimulants (used in the treatment of ADD/ADHD) Invega (paliperidone) Prozac (fluoxetine) Invega Sustenna Zoloft (sertraline) Adderall (amphetamine and Perseris (Risperidone injectable) dextroamphetamine) Latuda (lurasidone) MAOIs: Azstarys(dexmethylphenidate Rexulti (brexpiprazole) Emsam (selegiline) -

At-A-Glance: Psychotropic Drug Information for Children and Adolescents
At-A-Glance: Psychotropic Drug Information for Children and Adolescents Pediatric Dosage/ Drug Generic FDA Approval Serum Level Name Age/Indication when applicable Warnings and Precautions/Black Box Warnings Combination Antipsychotic/Antidepressant fluoxetine & 18 and older N/A: Pediatric Black Box Warning for fluoxetine/olanzapine olanzapine dosing is currently combination formula (marketed as Symbyax): Usage unavailable or not increased the risk of suicidal thinking and behaviors in applicable for this children and adolescents with major depressive disorder drug. and other psychiatric disorders. Other precautions for fluoxetine/olanzapine combination: Possibly unsafe during lactation. Avoid abrupt withdrawal. Antipsychotic Medications *Precautions which apply to all atypical or second generation antipsychotics (SGA): Neuroleptic Malignant Syndrome/Tardive Dyskinesia/ Hyperglycemia/ Diabetes Mellitus/ Weight Gain/ Akathisia/Dyslipidemia †Precautions which apply to all typical or first generation antipsychotics (FGA): Extrapyramidal symptoms/Tardive Dyskinesia aripiprazole * (SGA) 10 and older for 2-10 mg/kg/day Black Box Warning for aripiprazole: Not approved for bipolar disorder, depression in under age 18. Increased risk of suicidal manic, or mixed thinking and behavior in short-term studies in children episodes; 13 to 17 and adolescents with major depressive disorder and for schizophrenia other psychiatric conditions. and bipolar; 6 to 17 for irritability associated with autistic disorder asenapine* 18 and older N/A Black Box Warning for asenapine: Not approved for dementia-related psychosis. Increased mortality risk for elderly dementia patients due to cardiovascular or infectious events. chlorpromazine† 18 and older 0.25 mg/kg tid Other precautions for chlorpromazine: May alter cardiac (FGA) conduction; sedation; Neuroleptic Malignant Syndrome; weight gain. Use caution with renal disease, seizure disorders, and respiratory disease and in acute illness.