Proposal to Amend Appendix I Or II for CITES Cop16
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Article Look but Don't Lick! Find out How These Brightly Colored Frogs
The Power of Poison Look But Don’t Lick! GRADES K-2 NOTES FOR EDUCATORS Common Core State Standards: W.K-2.2, W.K-2.8 Have students read the article “Look but Don’t Lick!” Have them write notes RI.K-2.1, RI.K-2.2, RI.K-2.4, RI.K-2.7, RI.K-2.10 in the large right-hand margin. For example, they could underline key passages, paraphrase important information, or write down questions that New York State Science Core Curriculum: they have. LE 3.1a Next Generation Science Standards: If it is not possible to create color handouts, use a computer projector to PE 1-LS1-2 display the reading so that students can see the colorful frog photos. You DCI LS1.A: Structure and Function may also have them color their black and white copies to match the actual All organisms have external parts. Different colors. animals use their body parts in different ways to see, hear, grasp objects, protect Ask: themselves, move from place to place, and • What does it mean for an animal to be poisonous? (A: An animal is seek, find, and take in food, water and air. poisonous if its body contains a substance that is harmful or fatal to other Plants also have different parts (roots, animals.) stems, leaves, flowers, fruits) that help • How does being poisonous help the frogs in this article survive? (A: them survive and grow. Predators will not eat an animal that is poisonous to them. The frogs signal that they are poisonous by their bright colors, which warn predators not to eat them. -

Effects of Emerging Infectious Diseases on Amphibians: a Review of Experimental Studies
diversity Review Effects of Emerging Infectious Diseases on Amphibians: A Review of Experimental Studies Andrew R. Blaustein 1,*, Jenny Urbina 2 ID , Paul W. Snyder 1, Emily Reynolds 2 ID , Trang Dang 1 ID , Jason T. Hoverman 3 ID , Barbara Han 4 ID , Deanna H. Olson 5 ID , Catherine Searle 6 ID and Natalie M. Hambalek 1 1 Department of Integrative Biology, Oregon State University, Corvallis, OR 97331, USA; [email protected] (P.W.S.); [email protected] (T.D.); [email protected] (N.M.H.) 2 Environmental Sciences Graduate Program, Oregon State University, Corvallis, OR 97331, USA; [email protected] (J.U.); [email protected] (E.R.) 3 Department of Forestry and Natural Resources, Purdue University, West Lafayette, IN 47907, USA; [email protected] 4 Cary Institute of Ecosystem Studies, Millbrook, New York, NY 12545, USA; [email protected] 5 US Forest Service, Pacific Northwest Research Station, Corvallis, OR 97331, USA; [email protected] 6 Department of Biological Sciences, Purdue University, West Lafayette, IN 47907, USA; [email protected] * Correspondence [email protected]; Tel.: +1-541-737-5356 Received: 25 May 2018; Accepted: 27 July 2018; Published: 4 August 2018 Abstract: Numerous factors are contributing to the loss of biodiversity. These include complex effects of multiple abiotic and biotic stressors that may drive population losses. These losses are especially illustrated by amphibians, whose populations are declining worldwide. The causes of amphibian population declines are multifaceted and context-dependent. One major factor affecting amphibian populations is emerging infectious disease. Several pathogens and their associated diseases are especially significant contributors to amphibian population declines. -

Pumiliotoxin Metabolism and Molecular Physiology in a Poison Frog
bioRxiv preprint doi: https://doi.org/10.1101/2020.11.03.367524; this version posted November 5, 2020. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC 4.0 International license. 1 Pumiliotoxin metabolism and molecular physiology in a poison frog 2 3 Aurora Alvarez-Buylla1, Cheyenne Y. Payne1, Charles Vidoudez2, Sunia A. Trauger2 and Lauren 4 A. O’Connell*1 5 6 1 Department of Biology, Stanford University, Stanford, CA 94305, USA 7 2 Harvard Center for Mass Spectrometry, Harvard University, Cambridge, MA 02138, USA 8 9 Running title: Physiology of pumiliotoxin uptake 10 Word count (including methods): 2470 11 Word count (Abstract): 180 12 Key words: alkaloid, cytochrome P450, allopumiliotoxin, RNA sequencing, Dendrobatidae, 13 decahydroquinoline 14 15 * To whom correspondence should be addressed: 16 Lauren A. O’Connell 17 Department of Biology 18 Stanford University 19 371 Jane Stanford Way 20 Stanford, CA 94305 21 [email protected] 22 bioRxiv preprint doi: https://doi.org/10.1101/2020.11.03.367524; this version posted November 5, 2020. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC 4.0 International license. 23 ABSTRACT 24 Poison frogs bioaccumulate alkaloids for chemical defense from their arthropod diet. These 25 small molecules are sequestered from their gastrointestinal tract and transported to the skin for 26 storage. -

National Resource Material Green and Black Poison Frog (Dendrobates Auratus)
Indicative 10 Project National Resource Material Green and Black Poison frog (Dendrobates auratus) Michelle T. Christy and Win Kirkpatrick 2017 Department of Primary Industries and Regional Development 3 Baron-Hay Court, South Perth, WA 6151 An Invasive Animals CRC Project Contents Summary ............................................................................. 2 Key Messages ................................................................... 2 Classification ................................................................... 2 Common names ................................................................ 3 Biology and Ecology ................................................................ 3 Identification ................................................................... 3 Behaviours and Traits ......................................................... 4 Food and Foraging ............................................................. 4 Reproduction and Lifecycle ................................................. 5 Habitat ......................................................................... 5 Global Range ........................................................................ 5 Potential for Introduction ........................................................ 6 Potential for Eradication.......................................................... 7 Impacts ............................................................................... 7 Economic ........................................................................ 7 Environmental -

A Common Pumiliotoxin from Poison Frogs Exhibits Enantioselective Toxicity Against Mosquitoes
A common pumiliotoxin from poison frogs exhibits enantioselective toxicity against mosquitoes Paul J. Weldon*†, Matthew Kramer‡, Scott Gordon§¶, Thomas F. Spandeʈ, and John W. Daly†ʈ *Conservation and Research Center, Smithsonian Institution, 1500 Remount Road, Front Royal, VA 22630; ‡U.S. Department of Agriculture, Agricultural Research Service, Biometrical Consulting Service, Beltsville Agricultural Research Center, Beltsville, MD 20705; §Department of Entomology, Division of Communicable Diseases and Immunology, Walter Reed Army Institute of Research, Washington, DC 20307; and ʈLaboratory of Bioorganic Chemistry, National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health, Department of Health and Human Services, Building 8, Bethesda, MD 20892 Contributed by John W. Daly, September 29, 2006 (sent for review August 22, 2006) Neotropical poison frogs (Dendrobatidae) contain a variety of lipophilic alkaloids in their diffusely distributed cutaneous glands, including a major class of compounds known as pumiliotoxins. Pumiliotoxins are highly toxic and are believed to protect frogs against predators. Their potential activity against ectoparasites, however, has not been investigated. We tested female yellow fever mosquitoes (Aedes aegypti) for responses to 8-hydroxy-8- -methyl-6-(2-methylhexylidene)-1-azabicyclo[4.3.0]nonane, desig nated pumiliotoxin 251D [PTX (؉)-251D], a skin alkaloid present in all genera of dendrobatids and in other anurans, and to its unnatural enantiomer, PTX (؊)-251D. Both enantiomers of PTX 251D presented on silicone feeding membranes reduced landing Scheme 1. PTX (ϩ)-251D. and feeding by A. aegypti, but PTX (؉)-251D did so at lower concentrations. PTX (؉)-251D also induced toxicosis, shown when mosquitoes failed to fly off membranes. Similarly, mosquitoes and Minyobates, and in trace amounts in Phyllobates aurotaenia confined with copper wires coated with PTX (؉)-251D exhibited (12, 13) (Fig. -

Transcription Factors Mix1 and Vegt, Relocalization of Vegt Mrna, and Conserved Endoderm and Dorsal Specification in Frogs
Transcription factors Mix1 and VegT, relocalization of vegt mRNA, and conserved endoderm and dorsal specification in frogs Norihiro Sudoua,b, Andrés Garcés-Vásconezc, María A. López-Latorrec, Masanori Tairaa, and Eugenia M. del Pinoc,1 aDepartment of Biological Sciences, Graduate School of Science, University of Tokyo, Tokyo 113-0033, Japan; bDepartment of Anatomy, School of Medicine, Tokyo Women’s Medical University, Tokyo 162-8666, Japan; and cEscuela de Ciencias Biológicas, Pontificia Universidad Católica del Ecuador, Quito 170517, Ecuador Contributed by Eugenia M. del Pino, April 6, 2016 (sent for review December 16, 2015; reviewed by Igor B. Dawid and Jean-Pierre Saint-Jeannet) Protein expression of the transcription factor genes mix1 and vegt the posterior mesoderm (13). mix1 encodes a paired-like home- characterized the presumptive endoderm in embryos of the frogs odomain transcription factor expressed ubiquitously in the endo- Engystomops randi, Epipedobates machalilla, Gastrotheca riobambae, derm and mesoderm (14). Its expression is detected in the and Eleutherodactylus coqui,asinXenopus laevis embryos. Protein mesendoderm shortly after the mid-blastula transition and ex- VegT was detected in the animal hemisphere of the early blastula in pression peaks in the presumptive endoderm and mesoderm of all frogs, and only the animal pole was VegT-negative. This finding the X. laevis gastrula (14, 15). mix1 has not been sequenced in stimulated a vegt mRNA analysis in X. laevis eggs and embryos. vegt frogs other than Xenopus/Silurana. mRNA was detected in the animal region of X. laevis eggs and early The vegt ORF has been sequenced in the amphibians E. coqui Ec_vegt Rana pipiens Rp_vegt Ambystoma mexicanum embryos, in agreement with the VegT localization observed in the ( ), ( ), and (Am_vegt)(16–18) and partially sequenced in Epipedobates analyzed frogs. -

Thermal Adaptation of Amphibians in Tropical Mountains
Thermal adaptation of amphibians in tropical mountains. Consequences of global warming Adaptaciones térmicas de anfibios en montañas tropicales: consecuencias del calentamiento global Adaptacions tèrmiques d'amfibis en muntanyes tropicals: conseqüències de l'escalfament global Pol Pintanel Costa ADVERTIMENT. La consulta d’aquesta tesi queda condicionada a l’acceptació de les següents condicions d'ús: La difusió d’aquesta tesi per mitjà del servei TDX (www.tdx.cat) i a través del Dipòsit Digital de la UB (diposit.ub.edu) ha estat autoritzada pels titulars dels drets de propietat intel·lectual únicament per a usos privats emmarcats en activitats d’investigació i docència. No s’autoritza la seva reproducció amb finalitats de lucre ni la seva difusió i posada a disposició des d’un lloc aliè al servei TDX ni al Dipòsit Digital de la UB. No s’autoritza la presentació del seu contingut en una finestra o marc aliè a TDX o al Dipòsit Digital de la UB (framing). Aquesta reserva de drets afecta tant al resum de presentació de la tesi com als seus continguts. En la utilització o cita de parts de la tesi és obligat indicar el nom de la persona autora. ADVERTENCIA. La consulta de esta tesis queda condicionada a la aceptación de las siguientes condiciones de uso: La difusión de esta tesis por medio del servicio TDR (www.tdx.cat) y a través del Repositorio Digital de la UB (diposit.ub.edu) ha sido autorizada por los titulares de los derechos de propiedad intelectual únicamente para usos privados enmarcados en actividades de investigación y docencia. -

Advertisement Call of Pratt's Rocket Frog, Colostethus Pratti, from The
SALAMANDRA 56(4): 395–400 SALAMANDRA 30 October 2020 ISSN 0036–3375 Correspondence German Journal of Herpetology Correspondence Advertisement call of Pratt’s rocket frog, Colostethus pratti, from the western Andes of Colombia (Anura: Dendrobatidae) Juan David Jiménez-Bolaño1, Andrés Camilo Montes-Correa1, Franziska Leonhardt2 & Juan Manuel Renjifo1 1) Grupo de Investigación en Manejo & Conservación de Fauna, Flora & Ecosistemas Estratégicos Neotropicales (MIKU), Universidad del Magdalena, Santa Marta, Colombia 2) Museum of Zoology, Senckenberg Natural History Collections Dresden, A. B. Meyer Building, 01109 Dresden, Germany Corresponding author: Andrés Camilo Montes-Correa, e-mail: [email protected] Manuscript received: 22 July 2019 Accepted: 14 April 2020 by Stefan Lötters Northwestern South America is one of the biologically most phibian checklists (Cochran & Goin 1970, Ruiz-Car- diverse yet least known regions of the world. The Tropi- ranza et al. 1994, Acosta-Galvis 2000, Lynch & Suá- cal Andes and Chocó-Tumbes-Magdalena ecoregions, two rez-Mayorga 2004, Ballesteros-Correa et al. 2019). hotspots with high degrees of endemism and conservation The most recent information onC. pratti in Colombia con- priority (Myers et al. 2000, Rodríguez-Mahecha et al. cerns its altitudinal distribution, relative abundance, and 2004), are part of this region. Especially the Chocoan low- natural history in the northernmost part of the western lands and the western Andes of Colombia are areas with Andes, at the Serranía de San Jerónimo, Departamento de exceptional high amphibian diversity and many endemic Córdoba (Romero-Martínez et al. 2008, Ballesteros- taxa (e.g., Lynch et al. 1997, Lynch & Suárez-Mayorga Correa et al. 2019). To the best of our knowledge, there is 2004, Armesto & Señaris 2017). -

N.Orntates PUBLISHED by the AMERICAN MUSEUM of NATURAL HISTORY CENTRAL PARK WEST at 79TH STREET, NEW YORK, N.Y
AMERICAN MUSEUM N.orntates PUBLISHED BY THE AMERICAN MUSEUM OF NATURAL HISTORY CENTRAL PARK WEST AT 79TH STREET, NEW YORK, N.Y. 10024 Number 3068, 15 pp., 12 figures, 1 table June 11, 1993 A New Poison Frog from Manu INational Park, Southeastern Peru (Dendrobatidae, Epipedobates) LILY RODRIGUEZ' AND CHARLES W. MYERS2 ABSTRACT Epipedobates macero is a new species of den- ilar to a few other species occurring along the An- drobatid poison frog from lowland rain forest of dean front in eastern Peru, namely E. petersi and the Manu National Park, in the upper Madre de E. cainarachi, which differ in details ofcoloration, Dios drainage ofsoutheastern Peru. It is most sim- morphology, and vocalization. RESUMEN Epipedobates macero, especie nueva, es un den- del llano amaz6nico al pie de los Andes orientales drobatido venenoso de la selva pluvial baja del peruanos, a saber, E. petersi y E. cainarachi, las Parque Nacional del Manu, en el drenaje del Rio cuales difieren en detalles de coloracion, morfo- Alto Madre de Dios, al sudeste del Peru. Es similar logla, y vocalizacion. a otras dos especies que ocurren en los bosques ' Field Associate, Department of Herpetology and Ichthyology, American Museum of Natural History. Investi- gadora Asociada: Asociaci6n Peruana para la Conservaci6n de la Naturaleza (APECO), Parque Jos6 de Acosta 187, Lima 17, Perfi; and Museo de Historia Natural de la Universidad Mayor de San Marcos, apartado 140434, Lima 14, Peru. 2 Curator, Department of Herpetology and Ichthyology, American Museum of Natural History. Copyright © American Museum of Natural History 1993 ISSN 0003-0082 / Price $3.90 2 AMERICAN MUSEUM NOVITATES NO. -

Table 7: Species Changing IUCN Red List Status (2018-2019)
IUCN Red List version 2019-3: Table 7 Last Updated: 10 December 2019 Table 7: Species changing IUCN Red List Status (2018-2019) Published listings of a species' status may change for a variety of reasons (genuine improvement or deterioration in status; new information being available that was not known at the time of the previous assessment; taxonomic changes; corrections to mistakes made in previous assessments, etc. To help Red List users interpret the changes between the Red List updates, a summary of species that have changed category between 2018 (IUCN Red List version 2018-2) and 2019 (IUCN Red List version 2019-3) and the reasons for these changes is provided in the table below. IUCN Red List Categories: EX - Extinct, EW - Extinct in the Wild, CR - Critically Endangered [CR(PE) - Critically Endangered (Possibly Extinct), CR(PEW) - Critically Endangered (Possibly Extinct in the Wild)], EN - Endangered, VU - Vulnerable, LR/cd - Lower Risk/conservation dependent, NT - Near Threatened (includes LR/nt - Lower Risk/near threatened), DD - Data Deficient, LC - Least Concern (includes LR/lc - Lower Risk, least concern). Reasons for change: G - Genuine status change (genuine improvement or deterioration in the species' status); N - Non-genuine status change (i.e., status changes due to new information, improved knowledge of the criteria, incorrect data used previously, taxonomic revision, etc.); E - Previous listing was an Error. IUCN Red List IUCN Red Reason for Red List Scientific name Common name (2018) List (2019) change version Category -

Chromosome Analysis of Five Brazilian
c Indian Academy of Sciences RESEARCH ARTICLE Chromosome analysis of five Brazilian species of poison frogs (Anura: Dendrobatidae) PAULA CAMARGO RODRIGUES1, ODAIR AGUIAR2, FLÁVIA SERPIERI1, ALBERTINA PIMENTEL LIMA3, MASAO UETANEBARO4 and SHIRLEI MARIA RECCO-PIMENTEL1∗ 1Departamento de Anatomia, Biologia Celular e Fisiologia, Instituto de Biologia, Universidade Estadual de Campinas, 13083-863 Campinas, São Paulo, Brazil 2Departamento de Biociências, Universidade Federal de São Paulo, Campus Baixada Santista, 11060-001 Santos, São Paulo, Brazil 3Coordenadoria de Pesquisas em Ecologia, Instituto Nacional de Pesquisas do Amazonas, 69011-970 Manaus, Amazonas, Brazil 4Departamento de Biologia, Universidade Federal de Mato Grosso do Sul, 70070-900 Campo Grande, Mato Grosso do Sul, Brazil Abstract Dendrobatid frogs have undergone an extensive systematic reorganization based on recent molecular findings. The present work describes karyotypes of the Brazilian species Adelphobates castaneoticus, A. quinquevittatus, Ameerega picta, A. galactonotus and Dendrobates tinctorius which were compared to each other and with previously described related species. All karyotypes consisted of 2n = 18 chromosomes, except for A. picta which had 2n = 24. The karyotypes of the Adelphobates and D. tinctorius species were highly similar to each other and to the other 2n = 18 previously studied species, revealing conserved karyotypic characteristics in both genera. In recent phylogenetic studies, all Adelphobates species were grouped in a clade separated from the Dendrobates species. Thus, we hypothesized that their common karyotypic traits may have a distinct origin by chromosome rearrangements and mutations. In A. picta, with 2n = 24, chromosome features of pairs from 1 to 8 are shared with other previously karyotyped species within this genus. Hence, the A. -
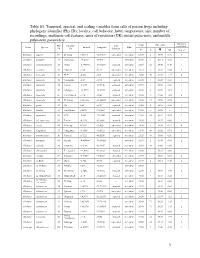
1 Table S1. Temporal, Spectral, and Scaling Variables from Calls Of
Table S1. Temporal, spectral, and scaling variables from calls of poison frogs including phylogeny identifier (Phy ID), locality, call behavior, habit, temperature, size, number of recordings, multinote call features, units of repetition (UR), initial pulse-note, and middle pulse-note parameters. Analyzed Phy Locality Call Temp SVL (mm) Genus Species Latitude Longitude Habit recordings ID ID Behavior °C N ! SD N of ♂ Allobates algorei 60 El Tama 7.65375 -72.19137 concealed terrestrial 23.50 8 18.90 0.70 3 Allobates brunneus 37 Guimaraes -15.2667 -55.5311 -- terrestrial 26.50 1 16.13 0.00 1 Allobates caeruleodactylus 48 Borba -4.398593 -59.60251 exposed terrestrial 25.60 12 15.50 0.40 1 Allobates crombiei 52 Altamira -3.65 -52.38 concealed terrestrial 24.10 2 18.10 0.04 2 Allobates femoralis 43 ECY -0.633 -76.5 concealed terrestrial 25.60 20 23.58 1.27 6 Allobates femoralis 46 Porongaba -8.67 -72.78 exposed terrestrial 25.00 1 25.38 0.00 1 Allobates femoralis 44 Leticia -4.2153 -69.9406 exposed terrestrial 25.50 1 20.90 0.00 1 Allobates femoralis 40 Albergue -12.8773 -71.3865 exposed terrestrial 26.00 6 21.98 2.18 1 Allobates femoralis 41 CAmazonico -12.6 -70.08 exposed terrestrial 26.00 12 22.43 1.06 4 Allobates femoralis 45 El Palmar 8.333333 -61.66667 concealed terrestrial 24.00 27 25.50 0.76 1 Allobates granti 49 FG 3.62 -53.17 exposed terrestrial 24.60 8 16.15 0.55 1 Allobates humilis 59 San Ramon 8.8678 -70.4861 concealed terrestrial 19.50 -- 21.80 -- 1 Allobates insperatus 54 ECY -0.633 -76.4005 exposed terrestrial 24.60 18 16.64 0.93 7 Allobates aff.