Observing National Nuclear Science Week
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Nobel Laureate George De Hevesy (1885-1966) - Universal Genius and Father of Nuclear Medicine Niese S* Am Silberblick 9, 01723 Wilsdruff, Germany
Open Access SAJ Biotechnology LETTER ISSN: 2375-6713 The Nobel Laureate George de Hevesy (1885-1966) - Universal Genius and Father of Nuclear Medicine Niese S* Am Silberblick 9, 01723 Wilsdruff, Germany *Corresponding author: Niese S, Am Silberblick 9, 01723 Wilsdruff, Germany, Tel: +49 35209 22849, E-mail: [email protected] Citation: Niese S, The Nobel Laureate George de Hevesy (1885-1966) - Universal Genius and Father of Nuclear Medicine. SAJ Biotechnol 5: 102 Article history: Received: 20 March 2018, Accepted: 29 March 2018, Published: 03 April 2018 Abstract The scientific work of the universal genius the Nobel Laureate George de Hevesy who has discovered and developed news in physics, chemistry, geology, biology and medicine is described. Special attention is given to his work in life science which he had done in the second half of his scientific career and was the base of the development of nuclear medicine. Keywords: George de Hevesy; Radionuclides; Nuclear Medicine Introduction George de Hevesy has founded Radioanalytical Chemistry and Nuclear Medicine, discovered the element hafnium and first separated stable isotopes. He was an inventor in many disciplines and his interest was not only focused on the development and refinement of methods, but also on the structure of matter and its changes: atoms, molecules, cells, organs, plants, animals, men and cosmic objects. He was working under complicated political situation in Europe in the 20th century. During his stay in Germany, Austria, Hungary, Switzerland, Denmark, and Sweden he wrote a lot papers in German. In 1962 he edited a large part of his articles in a collection where German papers are translated in English [1]. -

Chem 103, Section F0F Unit I
Lecture 4 - Observations that Led to the Chem 103, Section F0F Nuclear Model of the Atom Unit I - An Overview of Chemistry Dalton’s theory proposed that atoms were indivisible particles. Lecture 4 • By the late 19th century, this aspect of Dalton’s theory was being challenged. • Work with electricity lead to the discovery of the electron, • Some observations that led to the nuclear model as a particle that carried a negative charge. for the structure of the atom • The modern view of the atomic structure and the elements • Arranging the elements into a (periodic) table 2 Lecture 4 - Observations that Led to the Lecture 4 - Observations that Led to the Nuclear Model of the Atom Nuclear Model of the Atom The cathode ray In 1897, J.J. Thomson (1856-1940) studies how cathode rays • Cathode rays were shown to be electrons are affected by electric and magnetic fields • This allowed him to determine the mass/charge ration of an electron Cathode rays are released by metals at the cathode 3 4 Lecture 4 - Observations that Led to the Lecture 4 - Observations that Led to the Nuclear Model of the Atom Nuclear Model of the Atom In 1897, J.J. Thomson (1856-1940) studies how cathode rays In 1897, J.J. Thomson (1856-1940) studies how cathode rays are affected by electric and magnetic fields are affected by electric and magnetic fields • Thomson estimated that the mass of an electron was less • Thomson received the 1906 Nobel Prize in Physics for his that 1/1000 the mass of the lightest atom, hydrogen!! work. -

Otto Hahn Otto Hahn
R.N. 70269/98 Postal Registration No.: DL-SW-1/4082/15-17 ISSN : 0972-169X Date of posting: 26-27 of advance month Date of publication: 24 of advance month May 2017 Vol. 19 No. 8 Rs. 5.00 Otto Hahn Discoverer of Nuclear Fission Editorial: Consolidating 35 science communication activities in our country Otto Hahn: Discoverer of 34 Nuclear Fission Keep Your Eyes Healthy 31 Phenol: A Serious 30 Environmental Threat Accidental Discoveries in 28 Medical Science Cures for haemorrhoids— 24 Simple treatments and Surgeries Recent developments 21 in science and technology 36 Editorial Consolidating science communication activities in our country Dr. R. Gopichandran It is well known that the National Council of Science Museums of India’s leadership in science technology and innovation (STI) across the Ministry of Culture, Government of India, the National Institute the bilateral and multilateral framework also. The news feature service of Science Communication and Information Resources (NISCAIR) and the portal activity have well defined action plans to reach out to of CSIR, the National Council for Science and Technology fellow institutions and citizens with suitably embellished platform Communication (NCSTC) of the Department of Science and and opportunities for all to deliver together. Technology (DST), Government of India and Vigyan Prasar, also While these are interesting and extremely important, especially of DST, have been carrying out excellent science communication because they respond to the call to upscale and value add science activities over the years. It cannot be denied that the reach has been and technology communication, it is equally important to document quite significant collectively. -

Appendix E Nobel Prizes in Nuclear Science
Nuclear Science—A Guide to the Nuclear Science Wall Chart ©2018 Contemporary Physics Education Project (CPEP) Appendix E Nobel Prizes in Nuclear Science Many Nobel Prizes have been awarded for nuclear research and instrumentation. The field has spun off: particle physics, nuclear astrophysics, nuclear power reactors, nuclear medicine, and nuclear weapons. Understanding how the nucleus works and applying that knowledge to technology has been one of the most significant accomplishments of twentieth century scientific research. Each prize was awarded for physics unless otherwise noted. Name(s) Discovery Year Henri Becquerel, Pierre Discovered spontaneous radioactivity 1903 Curie, and Marie Curie Ernest Rutherford Work on the disintegration of the elements and 1908 chemistry of radioactive elements (chem) Marie Curie Discovery of radium and polonium 1911 (chem) Frederick Soddy Work on chemistry of radioactive substances 1921 including the origin and nature of radioactive (chem) isotopes Francis Aston Discovery of isotopes in many non-radioactive 1922 elements, also enunciated the whole-number rule of (chem) atomic masses Charles Wilson Development of the cloud chamber for detecting 1927 charged particles Harold Urey Discovery of heavy hydrogen (deuterium) 1934 (chem) Frederic Joliot and Synthesis of several new radioactive elements 1935 Irene Joliot-Curie (chem) James Chadwick Discovery of the neutron 1935 Carl David Anderson Discovery of the positron 1936 Enrico Fermi New radioactive elements produced by neutron 1938 irradiation Ernest Lawrence -

Facts and Figures 2013
Facts and Figures 201 3 Contents The University 2 World ranking 4 Academic pedigree 6 Areas of impact 8 Research power 10 Spin-outs 12 Income 14 Students 16 Graduate careers 18 Alumni 20 Faculties and Schools 22 Staff 24 Estates investment 26 Visitor attractions 28 Widening participation 30 At a glance 32 1 The University of Manchester Our Strategic Vision 2020 states our mission: “By 2020, The University of Manchester will be one of the top 25 research universities in the world, where all students enjoy a rewarding educational and wider experience; known worldwide as a place where the highest academic values and educational innovation are cherished; where research prospers and makes a real difference; and where the fruits of scholarship resonate throughout society.” Our core goals 1 World-class research 2 Outstanding learning and student experience 3 Social responsibility 2 3 World ranking The quality of our teaching and the impact of our research are the cornerstones of our success. 5 The Shanghai Jiao Tong University UK Academic Ranking of World ranking Universities assesses the best teaching and research universities, and in 2012 we were ranked 40th in the world. 7 World European UK European Year Ranking Ranking Ranking ranking 2012 40 7 5 2010 44 9 5 2005 53 12 6 2004* 78* 24* 9* 40 Source: 2012 Shanghai Jiao Tong University World Academic Ranking of World Universities ranking *2004 ranking refers to the Victoria University of Manchester prior to the merger with UMIST. 4 5 Academic pedigree Nobel laureates 1900 JJ Thomson , Physics (1906) We attract the highest calibre researchers and Ernest Rutherford , Chemistry (1908) teachers, boasting 25 Nobel Prize winners among 1910 William Lawrence Bragg , Physics (1915) current and former staff and students. -

Recollections and Reconstructions
@ ? Editor's Note: The Society's Nuclear Pioneer Citationfor 1977 goes not to an individual but to a distinguished group of individuals: those who took part in the epochal “ChicagoPile―experiment of Dec. 2, 1942, which produced thefirst successful atomicpile chain reaction. The 42 members ofthe Chicago Pilegroup are listed on page 591. One ofthose named, Harold M. Agnew, now Directorofthe Los Alamos Scient@flc Laboratory, is the Nuclear Pioneer Lecturerfor the 24th Annual Meeting. The guidingforce behind the Chicago Pile experiment was Enrico Fermi, recipient of the Nuclear Pioneer Citation of 1963. In the artick which follows, Laura Fermi recreates the excitement and mystery that surrounded the work ofher husband's research team in the early 1940s in Chicago. @. The FirstAtomic Pile: Recollections and Reconstructions by Laura Fermi @ . @ / - .-i@ ‘@:@ Enrico FermIat work, ca. 1942. As far as I can remember at the distance ofalmost 35 Perhaps not all husbands adhered as strictly as years, the first guests to arrive at our party on that Enrico to the rules of secrecy when talking to their beastly cold December night were the Zinns. Wally wives. He couldn't have been more tight-lipped. Once! and Jean. As they shook the snow off their coats, related some gossip to him: “Peoplesay that you stamping their feet on the floor, Wally turned to scientists at the Met Lab are seeking a cure for Enrico and said: “Congratulations!― I was surprised; cancer. .““Arewe?―he asked with his usual for Enrico had given me no hint that anything unusual imperturbable expression. -

MIT-NSE by the 1930S It Was Time to Discover Fission. We Knew About Isoto
Prelude to the Manhattan Project Michael V. Hynes – MIT-NSE By the 1930s it was time to discover fission. We knew about isotopes. The Curies and Becquerel had discovered radioactivity. Rutherford had discovered the nucleus. Chadwick had discovered the neutron. Einstein had discovered special relativity and the famous equation E=mc2. Bethe was even publishing about fusion as the source of energy for the Sun. So, it was time. By the 1930s a human drama was unfolding in Europe and in the Pacific – the rise of fascism. The events of this drama engulfed the world in war and created a need on all sides for weaponry that could defeat the enemy by whatever means. The intersection of scientific inevitability and war created the environment for the advent of nuclear weapons. Science was practiced was very differently in the 1920s and 1930s than today. In that era science was highly specialized and compartmentalized. If you were a metallurgist for example it was unlikely that you would have anything to do with a physicist – that all changed in the1940s. In 1933 Leo Szilard (Fig. 1), a Hungarian physicist who took refuge in London from Nazi Germany, read a paper by Rutherford that ridiculed the idea of getting energy from nuclear transmutations. Szilard realized that if you could find an element which is split by neutrons and which would emit two neutrons in the process, then a chain reaction could be started. This is the basic idea of a nuclear weapon -- to generate energy from the chain reaction. Szilard was a chemist by training and knew about the idea of a chemical chain reaction. -

Historical Background Prepared by Dr, Robin Chaplin Professor of Power Plant Engineering (Retired) University of New Brunswick
1 Historical Background prepared by Dr, Robin Chaplin Professor of Power Plant Engineering (retired) University of New Brunswick Summary: A review of the historical background for the development of nuclear energy is given to set the scene for the discussion of CANDU reactors. Table of Contents 1 Growth of Science and Technology......................................................................................... 2 2 Renowned Scientists............................................................................................................... 4 3 Significant Achievements........................................................................................................ 6 3.1 Niels Bohr........................................................................................................................ 6 3.2 James Chadwick .............................................................................................................. 6 3.3 Enrico Fermi .................................................................................................................... 6 4 Nuclear Fission........................................................................................................................ 7 5 Nuclear Energy........................................................................................................................ 7 6 Acknowledgments................................................................................................................... 8 List of Figures Figure 1 Timeline of significant discoveries -

Absolute Zero, Absolute Temperature. Absolute Zero Is the Lowest
Contents Radioactivity: The First Puzzles................................................ 1 The “Uranic Rays” of Henri Becquerel .......................................... 1 The Discovery ............................................................... 2 Is It Really Phosphorescence? .............................................. 4 What Is the Nature of the Radiation?....................................... 5 A Limited Impact on Scientists and the Public ............................ 6 Why 1896? .................................................................. 7 Was Radioactivity Discovered by Chance? ................................ 7 Polonium and Radium............................................................. 9 Marya Skłodowska .......................................................... 9 Pierre Curie .................................................................. 10 Polonium and Radium: Pierre and Marie Curie Invent Radiochemistry.. 11 Enigmas...................................................................... 14 Emanation from Thorium ......................................................... 17 Ernest Rutherford ........................................................... 17 Rutherford Studies Radioactivity: ˛-and ˇ-Rays.......................... 18 ˇ-Rays Are Electrons ....................................................... 19 Rutherford in Montreal: The Radiation of Thorium, the Exponential Decrease........................................... 19 “Induced” and “Excited” Radioactivity .................................... 20 Elster -
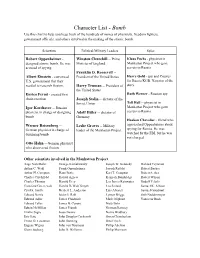
Character List
Character List - Bomb Use this chart to help you keep track of the hundreds of names of physicists, freedom fighters, government officials, and others involved in the making of the atomic bomb. Scientists Political/Military Leaders Spies Robert Oppenheimer - Winston Churchill -- Prime Klaus Fuchs - physicist in designed atomic bomb. He was Minister of England Manhattan Project who gave accused of spying. secrets to Russia Franklin D. Roosevelt -- Albert Einstein - convinced President of the United States Harry Gold - spy and Courier U.S. government that they for Russia KGB. Narrator of the needed to research fission. Harry Truman -- President of story the United States Enrico Fermi - created first Ruth Werner - Russian spy chain reaction Joseph Stalin -- dictator of the Tell Hall -- physicist in Soviet Union Igor Korchatov -- Russian Manhattan Project who gave physicist in charge of designing Adolf Hitler -- dictator of secrets to Russia bomb Germany Haakon Chevalier - friend who Werner Reisenberg -- Leslie Groves -- Military approached Oppenheimer about German physicist in charge of leader of the Manhattan Project spying for Russia. He was designing bomb watched by the FBI, but he was not charged. Otto Hahn -- German physicist who discovered fission Other scientists involved in the Manhattan Project: Aage Niels Bohr George Kistiakowsky Joseph W. Kennedy Richard Feynman Arthur C. Wahl Frank Oppenheimer Joseph Rotblat Robert Bacher Arthur H. Compton Hans Bethe Karl T. Compton Robert Serber Charles Critchfield Harold Agnew Kenneth Bainbridge Robert Wilson Charles Thomas Harold Urey Leo James Rainwater Rudolf Pelerls Crawford Greenewalt Harold DeWolf Smyth Leo Szilard Samuel K. Allison Cyril S. Smith Herbert L. Anderson Luis Alvarez Samuel Goudsmit Edward Norris Isidor I. -

Commentary & Notes Hans Bethe
J. Natn.Sci.Foundation Sri Lanka 2005 33(1): 57-58 COMMENTARY & NOTES HANS BETHE (1906-2005) Hans Albrecht Bethe who died on 6 March 2005 within the sun is hydrogen which is available in at the age of 98, was one of the most influential tremendous volume. Near the centre of the sun, and innovative theoretical physicists of our time. hydrogen nuclei can be squeezed under enormous pressure to become the element helium. If the Bethe was born in Strasbourg, Alsace- mass of hydrogen nucleus can be written as 1, Lorraine (then part of Germany) on 2 July 1906. Bethe showed that each time four nuclei of His father was a University physiologist and his hydrogen fused together as helium, their sum was mother Jewish, the daughter of a Strasbourg not equal to 4. The helium nucleus weighs about professor of Medicine. Bethe moved to Kiel and 0.7% less, or just 3.993 units of weight. It is this Frankfurt from where he went to Munich to carry missing 0.7% that comes out as a burst of explosive out research in theoretical physics under Arnold energy. The sun is so powerful that it pumps 4 Sommerfeld, who was not only a great teacher, million tons of hydrogen into pure energy every but an inspiring research supervisor as well. second. Einstein's famous equation, E = mc2, Bethe received his doctorate in 1928, for the work provides a clue to the massive energy that reaches he did on electron diffraction. He then went to the earth from the sun, when 4 million tons of the Cavendish laboratory in Cambridge on a hydrogen are multiplied by the figure c2 (square Rockefeller Fellowship, and Rome, before of the speed of light). -

Guide to the James Franck Papers 1882-1966
University of Chicago Library Guide to the James Franck Papers 1882-1966 © 2006 University of Chicago Library Table of Contents Acknowledgments 3 Descriptive Summary 3 Information on Use 3 Access 3 Citation 3 Biographical Note 4 Scope Note 15 Related Resources 21 Subject Headings 21 INVENTORY 22 Series I: Correspondence 22 Series II: Manuscripts 51 Subseries 1: Physics - work in Germany and Denmark, 1905-1934 51 Subseries 2: Physics - work in United States, 1935-1958 53 Subseries 3: Biophysics - work on Photosynthesis at Johns Hopkins, 1935-193855 Subseries 4: Biophysics - work on Photosynthesis at the University of Chicago,55 1938-48 Subseries 5: Biophysics - work on Photosynthesis after 1948 55 Subseries 6: General Articles and Talks on Science 71 Subseries 7: Papers by other scientists 72 Subseries 8: Notes, memoranda and fragments 76 Subseries 9: Atomic Scientists' Movement, 1944-1953 76 Subseries 10: Franck Memorial Symposium, May 12-13, 1966 79 Series III: Tape Recordings and Photographs 80 Subseries 1: Tape recordings 80 Subseries 2: Hertha Sponer's photograph album, Göttingen, 1920-1933 80 Series IV: Personal Documents and Memorabilia 90 Subseries 1: Documents 90 Subseries 2: Clippings 93 Subseries 3: Biographies and Obituaries 94 Subseries 4: Memorabilia; Scrolls, Certificates, Medals, Mementos 96 Series V: Robert Platzman's Editorial Papers for the "Selected Works of James98 Franck" Series VI: Addenda 103 Subseries 1: Correspondence between James Franck and his nephew and Dr. Heinz104 Kallman Subseries 2: Oversize 105 Descriptive Summary Identifier ICU.SPCL.FRANCK Title Franck, James. Papers Date 1882-1966 Size 20.5 linear feet (29 boxes) Repository Special Collections Research Center University of Chicago Library 1100 East 57th Street Chicago, Illinois 60637 U.S.A.