Full-Text (PDF)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

21.04.2021 Positive Patient Line List Raigarh.Xlsx
21.04.2021 Positive Patient Line list Raigarh 1 2021-04-21 S NO TestType AGE SEX Address District WARD NUMBER 20 VILLAGE SUKHDA JANJGIR 1 TruNat 27M CHAMPA JANJGIR CHAMPA 2 RTPCR 12 M NALWA PUNJIPATHARA TAMNAR RAIGARH WARD NUMBER 20 VILLAGE SUKHDA JANJGIR 3 TruNat 24F CHAMPA JANJGIR CHAMPA NUMBER 9 VILLAGE PASID BLOCK 4 TruNat 42F SARANGARH RAIGARH RAIGARH 5 RTPCR 47 M COLLECTION CENTER TAMNAR RAIGARH WARD NUMBER 28 PREM NAGAR 6 TruNat 35F CHAKRADHAR NAGAR RAIGARH RAIGARH 7 RTPCR 41 M NALWA TAMNAR RAIGARH 8 RTPCR 21 M TAMNAR TAMNAR RAIGARH 9 RTPCR 12 M NALWA TAMNAR RAIGARH 10 TruNat 40 M WARD NUMBER 25 LOCHAN NAGAR RAIGARH RAIGARH 11 RTPCR 42 M KONDKEL PORTA CABIN HINDALCO TAMNAR RAIGARH 12 RTPCR 40M NALWA TAMNAR RAIGARH 13 TruNat 37 F W N 25 LOCHAN NAGAR RAIGARH RAIGARH 14 RTPCR 48 M DHAURABHATHA TAMNAR RAIGARH 15 RTPCR 23 M JOBRO TAMNAR RAIGARH 16 RTPCR 22 M RANISAGER KHARSIA RAIGARH 17 TruNat 40 M RAIGARH RAIGARH 18 ANTIGEN 48 F VIKASH NAGAR GALI NO. 3 WARD NO. 39 RAIGARH 19 TruNat 28 M RAIGARH RAIGARH 20 RTPCR 56 M WORD NO 11 GHARGHODA RAIGARH 21 ANTIGEN 75 F BAJHINPALI JUTMIL W. N 32 RAIGARH RAIGARH 22 ANTIGEN 51 M DAROGAPARA RAIGARH RAIGARH 23 ANTIGEN 24 F BAJHINPALI JUTMIL RAIGARH RAIGARH 24 TruNat 11 F DULAMPUR BLOCK BARAMKELA RAIGARH 25 ANTIGEN 40 F RIWAPAR RAIGARH 26 ANTIGEN 42 M RIWAPAR RAIGARH 27 TruNat 42 F KANDOLA BLOCK BARAMKELA RAIGARH 28 ANTIGEN 55 F LENDHRA RAIGARH 29 TruNat 47 M KANDOLA BLOCK BARAMKELA RAIGARH 30 ANTIGEN 17 F LENDHRA RAIGARH 31 ANTIGEN 24 F BAWALI KUVA KOTRA ROAD RAIGARH RAIGARH 32 ANTIGEN 15 M LENDHRA RAIGARH 33 ANTIGEN 20 F PASID RAIGARH 34 ANTIGEN 40 F KEDAR RAIGARH 35 ANTIGEN 52 M KOTRA RAOD RAIHARH RAIGARH 36 ANTIGEN 25 F RAMGUDI TELIPARA WARD NO. -

Raigarh, Chhattisgarh
District Profile Raigarh, Chhattisgarh Raigarh is a major district in the state of Chhattisgarh in India. The headquarters of the district is located in the city of Raigarh. The total area of the district is 7086 sq. km. Raigarh district is divided into 10 Tehsils/ CD Blocks: Raigarh, Pusaur, Baramkela, Kharsiya, Gharghoda, Tamnar, Lallunga, Dharamjaigarh, Sa- rangarh. DEMOGRAPHY As per Census 2011, the total population of Raigarh is 14,93,984 which accounts for 5.85 percent of the total population of State. The percentage of urban population in Raigarh is 16.49 percent, which is lower than the state average of 23.24 percent. Out of the total population there are 750,278 males and 743,706 females in the district. This gives a sex ratio of 991.24 females per 1000 males. The decadal growth rate of population in Chhattisgarh is 22.59 percent, while Raigarh reports a 18.02 percent decadal increase in the population. The district population density is 211 in 2011. The Scheduled Caste population in the district is 15 percent while Scheduled Tribe comprises one third of the total population. LITERACY The overall literacy rate of Raigarh district is 73.26 percent while the male & female literacy rate is 83.49 and 63.02 percent respectively. At the block level, a considerable variation is noticeable in male-female literacy rate. Dharamjaigarh has the lowest literacy rate 62.43 percent, with 74.09 per- cent men and 50.90 percent women being literate. Raigarh block, subse- quently, has the highest literacy rates– among both males and females. -

Common Service Center List
CSC Profile Details Report as on 15-07-2015 SNo CSC ID District Name Block Name Village/CSC name Pincode Location VLE Name Address Line 1 Address Line 2 Address Line 3 E-mail Id Contact No 1 CG010100101 Durg Balod Karahibhadar 491227 Karahibhadar LALIT KUMAR SAHU vill post Karahibhadar block dist balod chhattisgarh [email protected] 8827309989 VILL & POST : NIPANI ,TAH : 2 CG010100102 Durg Balod Nipani 491227 Nipani MURLIDHAR C/O RAHUL COMUNICATION BALOD DISTRICT BALOD [email protected] 9424137413 3 CG010100103 Durg Balod Baghmara 491226 Baghmara KESHAL KUMAR SAHU Baghmara BLOCK-BALOD DURG C.G. [email protected] 9406116499 VILL & POST : JAGANNATHPUR ,TAH : 4 CG010100105 Durg Balod JAGANNATHPUR 491226 JAGANNATHPUR HEMANT KUMAR THAKUR JAGANNATHPUR C/O NIKHIL COMPUTER BALOD [email protected] 9479051538 5 CG010100106 Durg Balod Jhalmala 491226 Jhalmala SMT PRITI DESHMUKH VILL & POST : JHALMALA TAH : BALOD DIST:BALOD [email protected] 9406208255 6 CG010100107 Durg Balod LATABOD LATABOD DEKESHWAR PRASAD SAHU LATABOD [email protected] 9301172853 7 CG010100108 Durg Balod Piparchhedi 491226 PIPERCHEDI REKHA SAO Piparchhedi Block: Balod District:Balod [email protected] 9907125793 VILL & POST : JAGANNATHPUR JAGANNATHPUR.CSC@AISEC 8 CG010100109 Durg Balod SANKARAJ 491226 SANKARAJ HEMANT KUMAR THAKUR C/O NIKHIL COMPUTER ,TAH : BALOD DIST: BALOD TCSC.COM 9893483408 9 CG010100110 Durg Balod Bhediya Nawagaon 491226 Bhediya Nawagaon HULSI SAHU VILL & POST : BHEDIYA NAWAGAON BLOCK : BALOD DIST:BALOD [email protected] 9179037807 10 CG010100111 -

Chhattisgarh: Fastest Developing State of India Dr
Chhattisgarh: Fastest Developing State of India Dr. Raman Singh Chief Minister Our state Chhattisgarh was formed after a long political and cultural struggle. This region with rich cultural heritage, which was once known as Dakshin Kaushal, occupied 30 per cent of the undivided Madhya Pradesh. Despite being blessed with resources in abundance, it was one of the most backward and neglected areas. The demand for separate state was raised time and again, but it was our Ex-Prime Minister Mr. Atal Bihari Vajpayee, under whose tenure Chhattisgarh state came into existence on November 1, 2000. The state had to go through teething troubles in initial years, before its journey to create history commenced. Disappointed with the leadership during the initial few years after formation of state, people of Chhattisgarh entrusted me with their aspirations and hopes and elected me as Chief Minister of the state. It was undoubtedly a great responsibility. Chhattisgarh was superior to many states in terms of its natural and mineral resources. The state has nearly 44% of its area under forest cover. Its cultural, historical and archaeological glory has been incomparable. But sadly, the state was still among one of the most backward ones in the country and it used to get highlight mostly for the incidents of naxal violence. When I took charge as Chief Minister of Chhattisgarh, I had two major goals in my mind- 1) Optimal utilization of resources to ensure all-round socio-economic development and thereby, transform its image at national-level, 2) To uproot naxalism from state to ensure secured and peaceful environment in the affected areas. -

O.I.H. Government of India Ministry of Rural
O.I.H. GOVERNMENT OF INDIA MINISTRY OF RURAL DEVELOPMENT DEPARTMENT OF RURAL DEVELOPMENT LOK SABHA UNSTARRED QUESTION NO. 4374 ANSWERED ON 23.03.2021 PMGSY IN RAIGARH 4374. SHRIMATI GOMATI SAI: Will the Minister of RURAL DEVELOPMENT be pleased to state: (a) the details of the budget provision for reconstruction and repair of roads under Pradhan Mantri Gram Sadak Yojana (PMGSY) in the parliamentary constituency of Raigarh from the year 2015- 16 to 2020-21, year-wise; (b) the number of roads that have been built during the last five years upto January, 2021 under PMGSY in the parliamentary constituency of Raigarh; and (c) the number of roads that have been renewed and repaired upto January, 2021 in the said place including the details of the road-wise development, division-wise? ANSWER MINISTER OF STATE IN THE MINISTRY OF RURAL DEVELOPMENT (SADHVI NIRANJAN JYOTI) (a) As per PMGSY guidelines maintenance of roads constructed under the programme is the responsibility of the State Governments and all road works are covered by initial five year maintenance contracts to be entered into along with the construction contract, with the same contractor, as per the Standard Bidding Document. Maintenance funds to service the contract are required to be budgeted by the State Governments and placed at the disposal of the State Rural Roads Development Agencies (SRRDAs) in a separate maintenance account. On expiry of the 5 year post construction maintenance, PMGSY roads are required to be placed under Zonal maintenance contracts consisting of 5 year maintenance including renewal as per cycle, from time to time. -
TAMNAR Driven by JSPL's Vision and Ideologies, Jindal Power Limited
Home || Customer Sections || Contact Us Social World 1800-2082-008 NSE - Rs. 225.25 B Search About Us Business Products Sustainability InvestorsInvestors M Business IIndiia Chhattttiisgarrh Odiisha Jharrkhand IIntterrnattiionall Affrriica Oman ((Miiddlle Eastt)) Austtrralliia Techniicall Collllaborrattiion Home » Business » IndiaIndia » TAMNAR Driven by JSPL’’s vision and ideologies,ideologies, Jindal Power Limited (JPL), a subsidiary Raigarh of Jindal Steel & Power Limited, is a leading power company in India across the of Jindal Steel & Power Limited, is a leading power company in India across the Tamnar energy spectrum: thermal, hydro and renewable. JPL has been contributing significantly to the growing needs of power inin the country and itsits installedinstalled Raipur capacity is 3400 MW. Jindal Indus JPL commissioned thethe firstfirst unit of itsits 4x250 MW thermalthermal power plant inin thethe Raigarh district of Chhattisgarh inin 2007, becoming thethe firstfirst private playerr inin thethe country toto set up an IndependentIndependent Power Plant (IPP).(IPP). Since then,then, thisthis flagshipflagship has operated as a fullyfully integratedintegrated power plant at an industry-leading Plant Load Factor (PLF) of 93% (average for last seven years). Since itsits inception,inception, a 6.9 km longlong cross-country conveyor belt (the(the second longestlongest inin Asia), was installedinstalled toto feedfeed coal toto thethe plant. Among thethe other integratedintegrated facilitiesfacilities built by JPL are a dam toto feedfeed water toto thethe plant and a 258-km longlong 400-KV double circuit transmissiontransmission lineline toto pumppump thethe powerpower intointo thethe NationalNational Grid.Grid. Buoyed by thethe success of itsits 4x250 MW plant, JPL has furtherfurther expanded itsits presence inin Tamnar with a 2,400 MW (4X600(4X600 MW) thermalthermal power plant. -

Current Status of Lymphatic Filariasis in Sarangarh Tehsil, District Raigarh, Chhattisgarh
IOSR Journal of Pharmacy and Biological Sciences (IOSR-JPBS) e-ISSN: 2278-3008, p-ISSN:2319-7676. Volume 10, Issue 1 Ver. III (Jan -Feb. 2015), PP 01-03 www.iosrjournals.org Current Status of Lymphatic Filariasis in Sarangarh Tehsil, District Raigarh, Chhattisgarh Anita Pandey (Asst, Prof, Zoology) Govt. L. P.P. College Sarangarh, district-Raigarh C.G. Source of support-Nil, Tabel-1, Refrence-7 Govt.L.P.P. College Sara ngarh, Raigarh C.G Abstract: Local knowledge about lymphatic filariasis (LF) and their cause in villages of sarangarh tehsil, district Raigarh, C. G. Sarangarh tehsil is endemic for lymphatic filariasis. During July to November 2014, a lymphatic filariasis survey was carried out in six randomly selected villages and muhallas of sarangarh tehsil. Quantitative and qualitative methods were used for data collection. 202 individuals had disease manifestation of Lf. Female (55.44%) were more infected than male (44.55%), over all disease rate was (.70%) in all. Out of 202 diseased individuals, 57 male had hydrocele (28.21%), 126 had infected lower legs (62.36%) and 19 had infected upper arm (9.40%). Major causes of lymphatic filariasis is due to less awareness, unhygienic condition and late prevention. The present survey indicate that lymphatic filariasis is one of the major problem of public health in surveyed area. Background: Sarangarh tehsil has been known to be endemic for filariasis.. The present study was an epidemiological investigation carried out in villages (banjari, reda, hardi, timerlaga, godihari, kankvira) and muhallas of sarangarh from July to November 2014. Study Area: villages of Sarangarh tehsil, district Raigarh C.G. -
Published by Sampan Media Pvt. Ltd. for Chhattisgarh Tourism Board All Rights Reserved
Published by Sampan Media Pvt. Ltd. for Chhattisgarh Tourism Board All rights reserved. No part of this publication may be reproduced or transmitted in any form or by any means without the prior permission of the copyright owner. Details like telephone, fax numbers, opening hours, prices and travel information may change. The publisher is not responsible for error, if any. Copyright©2014 Chhattisgarh Tourism Board Published for Chhattisgarh Tourism Board by Sampan Media Pvt. Ltd. Regd. Office: 124, 3rd Floor, Shahpur Jat, Opp. Siri Fort Auditorium, New Delhi – 110049, Ph: 9560264447, [email protected] Contents Introducing Chhattisgarh 4 The Land and its History 5 Tribal Culture 16 Handicrafts 28 Cultural Traditions 39 Destinations 47 Raipur and Around 48 Bilaspur and Around 77 Bastar Division 104 National Parks and Sanctuaries 121 Practical Information 130 Introducing Chhattisgarh Chitrakote Fall The Land and its History hhattisgarh was a part of the state of Madhya Pradesh up till C2000, after which it was declared as a separate state with Raipur as the capital city. The state borders shares the states of Madhya Pradesh, Maharashtra, Andhra Pradesh, Odisha, Jharkhand and Uttar Pradesh. Located in central India, the state comprises of 27 districts. Chhattisgarh is endowed with rich cultural heritage and is a home of some of India’s ancient caves, waterfalls, The river side view temples, Buddhist sites, rock paintings, Chhattisgarh contains the wildlife and hill plateaus. source of one of the most important rivers of the South Historically Chhattisgarh shares Asian peninsula—the Mahanadi. its boundaries with many kingdoms. This river originates in a village The reason mentioned in the Imperial near Raipur. -

Ircon International Limited
IRCON INTERNATIONAL LIMITED DETAILED PROJECT REPORT‐CHHAL (CERL) CONSTRUCTION OF NEW COAL SIDING TAKING OFF FROM CHHAL STATION IN RAIGARH DISTRICT IN BILASPUR DIVISION OF SEC RAILWAY December ‐ 2018 Consultant HOWE ENGINEERING PROJECTS (INDIA) PVT. LTD. Office Address: Office No. 1003B, 10th Floor, BPTP Park, Centra, Sector‐30 NH‐8, Gurugram, Gurgaon, Haryana ‐ 122001 Telephone: 0124‐4740850, GST Regn. Number: 06AADCH4100N1ZS Registered Office: E‐102, Sanskar Appartment, Opp Karnavati Club, SG Highway, Satellite, Ahmedabad 380 015 Tel + 91 (79) 2555 6500, Fax + 91 (79) 2555 5603, www.howeindia.com CIN: U74140GJ2013PTC086504 Date: December 2018 Doc. No.: I‐527_IRCON_CERL_DPR_CHHAL Page 1 of 60 TABLE OF CONTENTS CHAPTER PAGE TITLE NO. NOS. 0 TABLE OF CONTENTS 1 to 1 I GENESIS OF THE PROPOSITION AND PROFILE OVERVIEW 2 to 11 OF THE ASSIGNMENT II PROJECT AT A GLANCE 12 to 16 III ANALYSIS & ASSESMENT OF COAL TRAFFIC 17 to 32 IV TOPOGRAPHY SURVEY 33 to 34 V CIVIL ENGINEERING (ALIGNMENT, BRIDGES & ROAD CROSSINGS AND OTHER 35 to 43 DETAILS) VI SYSTEM OF OPERATION 44 to 48 VII GEOTECHNICAL INVESTIGATION 49 to 49 VIII LAND PLANS 50 to 50 IX ELECTRICAL ENGINEERING 51 to 54 X SIGNAL AND TELECOMMUNICATION (S&T) ENGINEERING 55 to 55 XI MECHANICAL ENGINEERING 56 to 56 XII COST ESTIMATES 57 to 59 XIII LIST OF ANNEXURES 60 to 60 Detailed Project Report of Chhal (CERL) Date: December 2018 Doc. No.: I‐527_IRCON_CERL_DPR_CHHAL Page 2 of 60 CHAPTER ‐ 1: GENESIS OF THE PROPOSITION AND PROFILE OVERVIEW OF THE ASSIGNMENT INTRODUCTION: Bulk cargo, be it dry or liquid, like coal and crude oil play a crucial role in the economic development. -
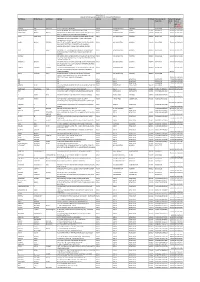
First Name Middle Name Last Name Address Country State
Biocon Limited Amount of unclimed and unpaid Interim dividend for FY 2010-11 First Name Middle Name Last Name Address Country State District PINCode Folio Number of Amount Proposed Securities Due(in Date of Rs.) transfer to IEPF (DD- MON-YYYY) JAGDISH DAS SHAH HUF CK 19/17 CHOWK VARANASI INDIA UTTAR PRADESH VARANASI BIO040743 150.00 03-JUN-2018 RADHESHYAM JUJU 8 A RATAN MAHAL APTS GHOD DOD ROAD SURAT INDIA GUJARAT SURAT 395001 BIO054721 150.00 03-JUN-2018 DAMAYANTI BHARAT BHATIA BNP PARIBASIAS OPERATIONS AKRUTI SOFTECH PARK ROAD INDIA MAHARASHTRA MUMBAI 400093 BIO001163 150.00 03-JUN-2018 NO 21 C CROSS ROAD MIDC ANDHERI E MUMBAI JYOTI SINGHANIA CO G.SUBRAHMANYAM, HEAD CAP MAR SER IDBI BANK LTD, INDIA MAHARASHTRA MUMBAI 400093 BIO011395 150.00 03-JUN-2018 ELEMACH BLDG PLOT 82.83 ROAD 7 STREET NO 15 MIDC, ANDHERI EAST, MUMBAI GOKUL MANOJ SEKSARIA IDBI LTD HEAD CAPITAL MARKET SERVIC CPU PLOT NO82/83 INDIA MAHARASHTRA MUMBAI 400093 BIO017966 150.00 03-JUN-2018 ROAD NO 7 STREET NO 15 OPP SPECIALITY RANBAXY LABORATORI ES MIDC ANDHERI (E) MUMBAI-4000093 DILIP P SHAH IDBI BANK, C.O. G.SUBRAHMANYAM HEAD CAP MARK SERV INDIA MAHARASHTRA MUMBAI 400093 BIO022473 150.00 03-JUN-2018 PLOT 82/83 ROAD 7 STREET NO 15 MIDC, ANDHERI.EAST, MUMBAI SURAKA IDBI BANK LTD C/O G SUBRAMANYAM HEAD CAPITAL MKT SER INDIA MAHARASHTRA MUMBAI 400093 BIO043568 150.00 03-JUN-2018 C P U PLOT NO 82/83 ROAD NO 7 ST NO 15 OPP RAMBAXY LAB ANDHERI MUMBAI (E) RAMANUJ MISHRA IDBI BANK LTD C/O G SUBRAHMANYAM HEAD CAP MARK SERV INDIA MAHARASHTRA MUMBAI 400093 BIO047663 150.00 03-JUN-2018 -

Chapter – I Introduction
CHAPTER – I INTRODUCTION WHAT IS BIODIVERSITY: Biological diversity or “biodiversity” has been defined as: “The variability among living organisms from all sources including Inter alia, Terrestrial, Marine and other Aquatic Ecosystems and the Ecological Complexes of which they are part; this includes diversity within species, between species, and of Ecosystems”. Diversity within species (or genetic diversity) refers to variability in the functional units of heredity present in any material of plant, animal, microbial or other origin. Species diversity is used to describe the variety of species - whether wild or domesticated) within a geographical area. Estimates of the total number of species (defined as a population of organisms which are able to interbreed freely under natural conditions) range from 2 to 100 million, though less than 1.5 million have actually been described. Ecosystem diversity refers to the enormous variety of plant, animal and micro organism communities and ecological processes that make them function. In short, biodiversity refers to the variety of life on earth. This variety provides the building blocks to adapt to changing environmental conditions in the future. The conservation of biodiversity is the fundamental to achieve sustainable development. It provides flexibility and options for our current (and future) use of natural resources. About 80% of the population in Chhattisgarh lives in rural areas, and a large part of this population, depends directly or indirectly on natural resources. Conservation of biodiversity is crucial for the sustainability of sectors as diverse as agriculture, forestry, fisheries, wildlife, industry, health, tourism, commerce, irrigation and power. Development of Chhattisgarh in future, will depend on the foundation provided by live resources, and conservation of biodiversity will ensure that this foundation remains strong. -

OHRJ-Vol II 3&4
ISSN NO : 0474- 7269 OHRJ, VOL. LVII No. 3&4 EDITORIAL BOARD Sri Manoranjan Panigrahi, IRS Chairman Principal Secretary to Govt., Odia Language, Literature and Culture Department, Govt. of Odisha. Sri Bijaya Kumar Nayak, IAS Vice Chairman Director & Addl. Secretary to Govt., Odia Language, Literature and Culture Department, Govt. of Odisha. Dr. K. K. Basa Member Professor and Head of Department of Anthropology, Utkal University, Vani Vihar, Bhubaneswar. Dr. Prafulla Kumar Mishra Member Vice Chancellor, North Odisha University, Baripada, Mayurbhanj. Dr. Sadasiba Pradhan (Retired) Member Professor of A I H, C & A Utkal University, Vani Vihar, Bhubaneswar. Dr. Patitapabana Mishra Member Retired Professor of History, Jyoti Vihar, Burla, Sambalpur. Dr. Sisir Kumar Panda Member Retired Professor of History, Bhanja Vihar, Berhampur. Dr. Chandi Prasad Nanda Member Professor of Modern History, Ravenshaw University, Cuttack. Dr. Jayanti Rath Member Convenor & Superintendent, Managing Editor Odisha State Museum, Bhubaneswar. ISSN NO : 0474- 7269 OHRJ, VOL. LVII No. 3&4 CONTENTS Topic Author Page 1. DATE OF JAYADEVA AND GITAGOVINDA Subas Pani 1 2. THREE NINETEENTH CENTURY SALE DEEDS Subrata Kumar Acharya 25 FROM GUNUPUR 3. THE BUGUḌĀ COPPER PLATE GRANT OF Dr. Bharati Pal 37 NEṬṬABHAÑJADEVA, YEAR 3 4. SIGNS AND SYMBOLS IN ROCK SHELTERS OF Dr. Sanjay Acharya 43 ODISHA : A STUDY ON ITS DESIGN, TYPE AND & Neha Sharma INTERPRETATIONS 5. AN EXCLUSIVE SURVEY REPORT ON THE Arabinda Bose 52 HISTORICAL SITES AND TEMPLES TRADITION IN NORTH ODISHA (Goloni, Kutilinga, Pedagodi, Kendua, Baripara, Mayurbhanj) 6. MESOLITHIC CULTURE OF JIRA RIVER VALLEY : Rajesh Kumar Sahu 64 A PRELIMINARY STUDY 7. DEVADASI OR MAHARI TRADITIONS OF THE Dr.