Oligodendrocyte Development and Regenerative Therapeutics in Multiple Sclerosis
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Induction of Motor Neurons by Sonic Hedgehog Is Independent of Floor Plate Differentiation Yasuto Tanabe, Henk Roelink and Thomas M
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Elsevier - Publisher Connector Induction of motor neurons by Sonic hedgehog is independent of floor plate differentiation Yasuto Tanabe, Henk Roelink and Thomas M. Jessell Howard Hughes Medical Institute, Deptartment of Biochemistry and Molecular Biophysics, Center for Neurobiology and Behavior, Columbia University, 701 West 168th Street, New York, New York 10032, USA. Background: The differentiation of floor plate cells and transfected with Shh induced both floor plate cells and motor neurons in the vertebrate neural tube appears to be motor neurons when grown in contact with neural plate induced by signals from the notochord. The secreted pro- explants, whereas only motor neurons were induced when tein encoded by the Sonic hedgehog (Shh) gene is expressed the explants were grown at a distance from Shh-trans- by axial midline cells and can induce floor plate cells in fected COS cells. Direct transfection of neural plate cells vivo and in vitro. Motor neurons can also be induced in with an Shh-expression construct induced both floor plate vitro by cells that synthesize Sonic hedgehog protein cells and motor neurons, with motor neuron differentia- (Shh). It remains unclear, however, if the motor-neuron- tion occurring prior to, or coincidentally with, floor plate inducing activity of Shh depends on the synthesis of a dis- differentiation. The induction of motor neurons appears, tinct signaling molecule by floor plate cells. To resolve therefore, not to depend on floor plate differentiation. this issue, we have developed an in vitro assay which Conclusions: The induction of motor neurons by uncouples the notochord-mediated induction of motor Shh does not depend on distinct floor-plate-derived neurons from floor plate differentiation, and have used signaling molecules. -

Oligodendrocytes in Development, Myelin Generation and Beyond
cells Review Oligodendrocytes in Development, Myelin Generation and Beyond Sarah Kuhn y, Laura Gritti y, Daniel Crooks and Yvonne Dombrowski * Wellcome-Wolfson Institute for Experimental Medicine, Queen’s University Belfast, Belfast BT9 7BL, UK; [email protected] (S.K.); [email protected] (L.G.); [email protected] (D.C.) * Correspondence: [email protected]; Tel.: +0044-28-9097-6127 These authors contributed equally. y Received: 15 October 2019; Accepted: 7 November 2019; Published: 12 November 2019 Abstract: Oligodendrocytes are the myelinating cells of the central nervous system (CNS) that are generated from oligodendrocyte progenitor cells (OPC). OPC are distributed throughout the CNS and represent a pool of migratory and proliferative adult progenitor cells that can differentiate into oligodendrocytes. The central function of oligodendrocytes is to generate myelin, which is an extended membrane from the cell that wraps tightly around axons. Due to this energy consuming process and the associated high metabolic turnover oligodendrocytes are vulnerable to cytotoxic and excitotoxic factors. Oligodendrocyte pathology is therefore evident in a range of disorders including multiple sclerosis, schizophrenia and Alzheimer’s disease. Deceased oligodendrocytes can be replenished from the adult OPC pool and lost myelin can be regenerated during remyelination, which can prevent axonal degeneration and can restore function. Cell population studies have recently identified novel immunomodulatory functions of oligodendrocytes, the implications of which, e.g., for diseases with primary oligodendrocyte pathology, are not yet clear. Here, we review the journey of oligodendrocytes from the embryonic stage to their role in homeostasis and their fate in disease. We will also discuss the most common models used to study oligodendrocytes and describe newly discovered functions of oligodendrocytes. -

Investigation of Candidate Genes and Mechanisms Underlying Obesity
Prashanth et al. BMC Endocrine Disorders (2021) 21:80 https://doi.org/10.1186/s12902-021-00718-5 RESEARCH ARTICLE Open Access Investigation of candidate genes and mechanisms underlying obesity associated type 2 diabetes mellitus using bioinformatics analysis and screening of small drug molecules G. Prashanth1 , Basavaraj Vastrad2 , Anandkumar Tengli3 , Chanabasayya Vastrad4* and Iranna Kotturshetti5 Abstract Background: Obesity associated type 2 diabetes mellitus is a metabolic disorder ; however, the etiology of obesity associated type 2 diabetes mellitus remains largely unknown. There is an urgent need to further broaden the understanding of the molecular mechanism associated in obesity associated type 2 diabetes mellitus. Methods: To screen the differentially expressed genes (DEGs) that might play essential roles in obesity associated type 2 diabetes mellitus, the publicly available expression profiling by high throughput sequencing data (GSE143319) was downloaded and screened for DEGs. Then, Gene Ontology (GO) and REACTOME pathway enrichment analysis were performed. The protein - protein interaction network, miRNA - target genes regulatory network and TF-target gene regulatory network were constructed and analyzed for identification of hub and target genes. The hub genes were validated by receiver operating characteristic (ROC) curve analysis and RT- PCR analysis. Finally, a molecular docking study was performed on over expressed proteins to predict the target small drug molecules. Results: A total of 820 DEGs were identified between -

Sonic Hedgehog Induces the Lateral Floor Plate
Development 129, 4785-4796 (2002) 4785 Printed in Great Britain © The Company of Biologists Limited 2002 DEV2912 Dual origin of the floor plate in the avian embryo Jean-Baptiste Charrier, Françoise Lapointe, Nicole M. Le Douarin and Marie-Aimée Teillet* Institut d’Embryologie Cellulaire et Moléculaire, CNRS and Collège de France, UMR 7128, 49bis Avenue de la Belle Gabrielle, 94736 Nogent-sur-Marne Cedex, France *Author for correspondence (e-mail: [email protected]) Accepted 30 July 2002 SUMMARY Molecular analysis carried out on quail-chick chimeras, in development, one can experimentally obtain a complete which quail Hensen’s node was substituted for its chick floor plate in the neural epithelium by the inductive action counterpart at the five- to six-somite stage (ss), showed that of either a notochord or a MFP. The competence of the the floor plate of the avian neural tube is composed of neuroepithelium to respond to notochord or MFP signals is distinct areas: (1) a median one (medial floor plate or MFP) restricted to a short time window, as only the posterior-most derived from Hensen’s node and characterised by the same region of the neural plate of embryos younger than 15 ss is gene expression pattern as the node cells (i.e. expression of able to differentiate a complete floor plate comprising MFP HNF3β and Shh to the exclusion of genes early expressed and LFP. Moreover, MFP differentiation requires between in the neural ectoderm such as CSox1); and (2) lateral 4 and 5 days of exposure to the inducing tissues. -

Cell Reprogramming Technologies for Treatment And
CELL REPROGRAMMING TECHNOLOGIES FOR TREATMENT AND UNDERSTANDING OF GENETIC DISORDERS OF MYELIN by ANGELA MARIE LAGER Submitted in partial fulfillment of the requirements for the degree of Doctor of Philosophy Thesis advisor: Paul J Tesar, PhD Department of Genetics and Genome Sciences CASE WESTERN RESERVE UNIVERSITY May 2015 CASE WESTERN RESERVE UNIVERSITY SCHOOL OF GRADUATE STUDIES We hereby approve the thesis/dissertation of Angela Marie Lager Candidate for the Doctor of Philosophy degree*. (signed) Ronald A Conlon, PhD (Committee Chair) Paul J Tesar, PhD (Advisor) Craig A Hodges, PhD Warren J Alilain, PhD (date) 31 March 2015 *We also certify that written approval has been obtained from any proprietary material contained therein. TABLE OF CONTENTS Table of Contents……………………………………………………………………….1 List of Figures……………………………………………………………………………4 Acknowledgements……………………………………………………………………..7 Abstract…………………………………………………………………………………..8 Chapter 1: Introduction and Background………………………………………..11 1.1 Overview of mammalian oligodendrocyte development in the spinal cord and myelination of the central nervous system…………………..11 1.1.1 Introduction……………………………………………………..11 1.1.2 The establishment of the neuroectoderm and ventral formation of the neural tube…………………………………..12 1.1.3 Ventral patterning of the neural tube and specification of the pMN domain in the spinal cord……………………………….15 1.1.4 Oligodendrocyte progenitor cell production through the process of gliogenesis ………………………………………..16 1.1.5 Oligodendrocyte progenitor cell to oligodendrocyte differentiation…………………………………………………...22 -

Cellular and Molecular Signatures in the Disease Tissue of Early
Cellular and Molecular Signatures in the Disease Tissue of Early Rheumatoid Arthritis Stratify Clinical Response to csDMARD-Therapy and Predict Radiographic Progression Frances Humby1,* Myles Lewis1,* Nandhini Ramamoorthi2, Jason Hackney3, Michael Barnes1, Michele Bombardieri1, Francesca Setiadi2, Stephen Kelly1, Fabiola Bene1, Maria di Cicco1, Sudeh Riahi1, Vidalba Rocher-Ros1, Nora Ng1, Ilias Lazorou1, Rebecca E. Hands1, Desiree van der Heijde4, Robert Landewé5, Annette van der Helm-van Mil4, Alberto Cauli6, Iain B. McInnes7, Christopher D. Buckley8, Ernest Choy9, Peter Taylor10, Michael J. Townsend2 & Costantino Pitzalis1 1Centre for Experimental Medicine and Rheumatology, William Harvey Research Institute, Barts and The London School of Medicine and Dentistry, Queen Mary University of London, Charterhouse Square, London EC1M 6BQ, UK. Departments of 2Biomarker Discovery OMNI, 3Bioinformatics and Computational Biology, Genentech Research and Early Development, South San Francisco, California 94080 USA 4Department of Rheumatology, Leiden University Medical Center, The Netherlands 5Department of Clinical Immunology & Rheumatology, Amsterdam Rheumatology & Immunology Center, Amsterdam, The Netherlands 6Rheumatology Unit, Department of Medical Sciences, Policlinico of the University of Cagliari, Cagliari, Italy 7Institute of Infection, Immunity and Inflammation, University of Glasgow, Glasgow G12 8TA, UK 8Rheumatology Research Group, Institute of Inflammation and Ageing (IIA), University of Birmingham, Birmingham B15 2WB, UK 9Institute of -

Induction of Floor Plate Differentiation by Contact-Dependent
Development 117, 205-218 (1993) 205 Printed in Great Britain © The Company of Biologists Limited 1993 Induction of floor plate differentiation by contact-dependent, homeogenetic signals Marysia Placzek1, Thomas M. Jessell2 and Jane Dodd1,* 1Department of Physiology and Cellular Biophysics, 2Department of Biochemistry and Molecular Biophysics and Howard Hughes Medical Institute, and 1,2Center for Neurobiology and Behavior, Columbia University, New York, New York 10032, USA *Author for correspondence SUMMARY The floor plate is located at the ventral midline of the derive from medial floor plate cells. The response of neural tube and has been implicated in neural cell pat- neural plate cells to inductive signals declines with terning and axon guidance. To address the cellular embryonic age, suggesting that the mediolateral extent mechanisms involved in floor plate differentiation, we of the floor plate is limited by a loss of competence of have used an assay that monitors the expression of floor- neural cells. The rostral boundary of the floor plate at plate-specific antigens in neural plate explants cultured the midbrain-forebrain junction appears to result from in the presence of inducing tissues. Contact-mediated the lack of inducing activity in prechordal mesoderm signals from both the notochord and the floor plate act and the inability of rostral neural plate cells to respond directly on neural plate cells to induce floor plate differ- to inductive signals. entiation. Floor plate induction is initiated medially by a signal from the notochord, but appears to be propa- gated to more lateral cells by homeogenetic signals that Key words: floor plate, notochord, neural plate, induction. -

United States Patent (19) (11) 4,232,002 Nogrady 45) Nov
United States Patent (19) (11) 4,232,002 Nogrady 45) Nov. 4, 1980 (54) PROCEDURES AND PHARMACEUTICAL (56) References Cited PRODUCTS FOR USE IN THE PUBLICATIONS ADMINISTRATION OF ANTHISTAMINES American Hospital Formulary Service, 1966, 4:00 Anti (75. Inventor: Stephen G. Nogrady, Sully, near histamine Drugs, Penarth, Great Britain Primary Examiner-Stanley J. Friedman Attorney, Agent, or Firm-Young & Thompson 73) Assignee: The Welsh National School of Medicine, Penarth, Great Britain 57 ABSTRACT An antihistamine of the benzhydrylether, alkylamine, or (21) Appl. No.: 965,171 benzocyloheptatiophene class is suitable for use in the therapeutic treatment or prophylaxis of reversible air (22 Filed: Nov.30, 1978 ways obstruction by inhalation. The antihistamine may be clemastine, chlorpheniramine or ketotifen and may (30) Foreign Application Priority Data be in the form of a composition in admixture with a diluent. The antihistamine can be administered from a Dec. 1, 1977 GB) United Kingdom ..................... 5.0020 pharmaceutical inhalation device which is designed to 51 Int. Cl. ......................... A61L 9/04; A61 K9/04; administer a dosage unit of the antihistamine. The inha A61K 31/44 lation device can be in the form of a pressurized aerosol 52 U.S. C. ........................................ 424/45; 424/46; inhaler or a dry powder insufflator. 424/263 58) Field of Search ............................ 424/263, 46, 45 5 Claims, No Drawings 4,232,002 1. 2 inhalation provides the equivalent of 0.1 to 5 mg. of PROCEDURES AND PHARMACEUTICAL clemastine, or 0.05 to 2.5 mg. of chlorpheniramine. The PRODUCTS FOR USE IN THE ADMINISTRATION drug may be inhaled in the form of a mist or nebulized OF ANTHISTAMINES spray, or as a cloud of fine solid particles, and may be inhaled from a variety of inhaler devices. -

Investigation of the Underlying Hub Genes and Molexular Pathogensis in Gastric Cancer by Integrated Bioinformatic Analyses
bioRxiv preprint doi: https://doi.org/10.1101/2020.12.20.423656; this version posted December 22, 2020. The copyright holder for this preprint (which was not certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission. Investigation of the underlying hub genes and molexular pathogensis in gastric cancer by integrated bioinformatic analyses Basavaraj Vastrad1, Chanabasayya Vastrad*2 1. Department of Biochemistry, Basaveshwar College of Pharmacy, Gadag, Karnataka 582103, India. 2. Biostatistics and Bioinformatics, Chanabasava Nilaya, Bharthinagar, Dharwad 580001, Karanataka, India. * Chanabasayya Vastrad [email protected] Ph: +919480073398 Chanabasava Nilaya, Bharthinagar, Dharwad 580001 , Karanataka, India bioRxiv preprint doi: https://doi.org/10.1101/2020.12.20.423656; this version posted December 22, 2020. The copyright holder for this preprint (which was not certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission. Abstract The high mortality rate of gastric cancer (GC) is in part due to the absence of initial disclosure of its biomarkers. The recognition of important genes associated in GC is therefore recommended to advance clinical prognosis, diagnosis and and treatment outcomes. The current investigation used the microarray dataset GSE113255 RNA seq data from the Gene Expression Omnibus database to diagnose differentially expressed genes (DEGs). Pathway and gene ontology enrichment analyses were performed, and a proteinprotein interaction network, modules, target genes - miRNA regulatory network and target genes - TF regulatory network were constructed and analyzed. Finally, validation of hub genes was performed. The 1008 DEGs identified consisted of 505 up regulated genes and 503 down regulated genes. -

Myelin Impairs CNS Remyelination by Inhibiting Oligodendrocyte Precursor Cell Differentiation
328 • The Journal of Neuroscience, January 4, 2006 • 26(1):328–332 Brief Communication Myelin Impairs CNS Remyelination by Inhibiting Oligodendrocyte Precursor Cell Differentiation Mark R. Kotter, Wen-Wu Li, Chao Zhao, and Robin J. M. Franklin Cambridge Centre for Brain Repair and Department of Veterinary Medicine, University of Cambridge, Cambridge CB3 0ES, United Kingdom Demyelination in the adult CNS can be followed by extensive repair. However, in multiple sclerosis, the differentiation of oligodendrocyte lineage cells present in demyelinated lesions is often inhibited by unknown factors. In this study, we test whether myelin debris, a feature of demyelinated lesions and an in vitro inhibitor of oligodendrocyte precursor differentiation, affects remyelination efficiency. Focal demyelinating lesions were created in the adult rat brainstem, and the naturally generated myelin debris was augmented by the addition of purified myelin. After quantification of myelin basic protein mRNA expression from lesion material obtained by laser capture micro- dissection and supported by histological data, we found a significant impairment of remyelination, attributable to an arrest of the differentiation and not the recruitment of oligodendrocyte precursor cells. These data identify myelin as an inhibitor of remyelination as well as its well documented inhibition of axon regeneration. Key words: regeneration; remyelination; oligodendrocyte precursor cell; myelin; differentiation; macrophage Introduction of myelin debris to efficient remyelination and experimental The adult CNS contains a precursor cell population that responds studies in which delayed phagocytosis of myelin debris is associ- to demyelination by undergoing rapid proliferation, migration, ated with a simultaneous delay of OPC differentiation (Kotter et and differentiation into new oligodendrocytes (Horner et al., al., 2005). -

Myelin Biogenesis Is Associated with Pathological Ultrastructure That Is
bioRxiv preprint doi: https://doi.org/10.1101/2021.02.02.429485; this version posted February 4, 2021. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY 4.0 International license. 1 Myelin biogenesis is associated with pathological ultrastructure that 2 is resolved by microglia during development 3 4 5 Minou Djannatian1,2*, Ulrich Weikert3, Shima Safaiyan1,2, Christoph Wrede4, Cassandra 6 Deichsel1,2, Georg Kislinger1,2, Torben Ruhwedel3, Douglas S. Campbell5, Tjakko van Ham6, 7 Bettina Schmid2, Jan Hegermann4, Wiebke Möbius3, Martina Schifferer2,7, Mikael Simons1,2,7* 8 9 1Institute of Neuronal Cell Biology, Technical University Munich, 80802 Munich, Germany 10 2German Center for Neurodegenerative Diseases (DZNE), 81377 Munich, Germany 11 3Max-Planck Institute of Experimental Medicine, 37075 Göttingen, Germany 12 4Institute of Functional and Applied Anatomy, Research Core Unit Electron Microscopy, 13 Hannover Medical School, 30625, Hannover, Germany 14 5Department of Neuronal Remodeling, Graduate School of Pharmaceutical Sciences, Kyoto 15 University, Sakyo-ku, Kyoto 606-8501, Japan. 16 6Department of Clinical Genetics, Erasmus MC, University Medical Center Rotterdam, 3015 CN, 17 the Netherlands 18 7Munich Cluster of Systems Neurology (SyNergy), 81377 Munich, Germany 19 20 *Correspondence: [email protected] or [email protected] 21 Keywords 22 Myelination, degeneration, phagocytosis, microglia, oligodendrocytes, phosphatidylserine 23 24 1 bioRxiv preprint doi: https://doi.org/10.1101/2021.02.02.429485; this version posted February 4, 2021. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. -
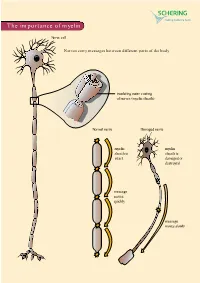
The Importance of Myelin
The importance of myelin Nerve cell Nerves carry messages between different parts of the body insulating outer coating of nerves (myelin sheath) Normal nerve Damaged nerve myelin myelin sheath is sheath is intact damaged or destroyed message moves quickly message moves slowly Nerve cells transmit impulses Nerve cells have a long, thin, flexible fibre that transmits impulses. These impulses are electrical signals that travel along the length of the nerve. Nerve fibres are long to enable impulses to travel between distant parts of the body, such as the spinal cord and leg muscles. Myelin speeds up impulses Most nerve fibres are surrounded by an insulating, fatty sheath called myelin, which acts to speed up impulses. The myelin sheath contains periodic breaks called nodes of Ranvier. By jumping from node to node, the impulse can travel much more quickly than if it had to travel along the entire length of the nerve fibre. Myelinated nerves can transmit a signal at speeds as high as 100 metres per second – as fast as a Formula One racing car. normal damaged nerve nerve Loss of myelin leads to a variety of symptoms If the myelin sheath surrounding nerve fibres is damaged or destroyed, transmission of nerve impulses is slowed or blocked. The impulse now has to flow continuously along the whole nerve fibre – a process that is much slower than jumping from node to node. Loss of myelin can also lead to ‘short-circuiting’ of nerve impulses. An area where myelin has been destroyed is called a lesion or plaque. This slowing and ‘short-circuiting’ of nerve impulses by lesions leads to a variety of symptoms related to nervous system activity.