Forest Insect and Disease Rapid Response Plan
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Ecology, Behavior, and Biological Control Potential of Hymenopteran Parasitoids of Woodwasps (Hymenoptera: Siricidae) in North America
REVIEW:BIOLOGICAL CONTROL-PARASITOIDS &PREDATORS The Ecology, Behavior, and Biological Control Potential of Hymenopteran Parasitoids of Woodwasps (Hymenoptera: Siricidae) in North America 1 DAVID R. COYLE AND KAMAL J. K. GANDHI Daniel B. Warnell School of Forestry and Natural Resources, University of Georgia, Athens, GA 30602 Environ. Entomol. 41(4): 731Ð749 (2012); DOI: http://dx.doi.org/10.1603/EN11280 ABSTRACT Native and exotic siricid wasps (Hymenoptera: Siricidae) can be ecologically and/or economically important woodboring insects in forests worldwide. In particular, Sirex noctilio (F.), a Eurasian species that recently has been introduced to North America, has caused pine tree (Pinus spp.) mortality in its non-native range in the southern hemisphere. Native siricid wasps are known to have a rich complex of hymenopteran parasitoids that may provide some biological control pressure on S. noctilio as it continues to expand its range in North America. We reviewed ecological information about the hymenopteran parasitoids of siricids in North America north of Mexico, including their distribution, life cycle, seasonal phenology, and impacts on native siricid hosts with some potential efÞcacy as biological control agents for S. noctilio. Literature review indicated that in the hymenop- teran families Stephanidae, Ibaliidae, and Ichneumonidae, there are Þve genera and 26 species and subspecies of native parasitoids documented from 16 native siricids reported from 110 tree host species. Among parasitoids that attack the siricid subfamily Siricinae, Ibalia leucospoides ensiger (Norton), Rhyssa persuasoria (L.), and Megarhyssa nortoni (Cresson) were associated with the greatest number of siricid and tree species. These three species, along with R. lineolata (Kirby), are the most widely distributed Siricinae parasitoid species in the eastern and western forests of North America. -

Research Collection
Research Collection Doctoral Thesis Studies on Venturiaceae on Rosaceous plants Author(s): Menon, Radha Publication Date: 1956 Permanent Link: https://doi.org/10.3929/ethz-a-000092066 Rights / License: In Copyright - Non-Commercial Use Permitted This page was generated automatically upon download from the ETH Zurich Research Collection. For more information please consult the Terms of use. ETH Library Diss ETH Prom. Nr. 2585 B Studies on Venturiaceae on Rosaceous Plants THESIS PRESENTED TO THE SWISS FEDERAL INSTITUTE OF TECHNOLOGY ZURICH FOR THE DEGREE OF DOCTOR OF NATURAL SCIENCES BY RADHA MENON at CITIZEN OF Ser\ INDIA Accepted on the recommendation of Prof. Dr. E. Gaumann and Prof. Dr. A. Frey-Wyssling 19 5 6 Druck von A. W. Hayn's Erben, Berlin SO 36 Veroffentlicht in „Phytopathologische Zcitschrift" Band 27, Heft 2, Seite 117 bis 146 (1956) Verlag Paul Parey, Berlin und Hamburg From the Department of special Botany of the Swiss Federal Institute of Technology in Zurich Director: Prof. Dr. E. Gdumann Studies on Venturiaceae on Rosaceous Plants By Radha Menon With 10 Figures Contents: I. General Introduction. A. Venturiaceae. B. Venturiaceae on Rosaceae: 1) Venturia, 2) Coleroa, 3) Gibbera, 4) Xenomeris, 5) Apiosporina. — II. Experimental Part. A. Cultural Studies. B. Inoculation Experiments: 1) Introduction, 2) Inoculation Studies, 3) Results, 4) Conclusions. — III. Morphological and Cultural Studies. A. Genus Venturia: 1) Venturia inaequalis, 2) Venturia tomentosae, 3) Venturia pirina, 4) Venturia pruni-cerasi, 5) Venturia Mullcri, 6) Venturia potentillae, 7) Venturia palustris, 8) Venturia alchemillae. — Appendix: Fusicladium eriobotryae. — B. Genus Coleroa: Coleroa chac- tomium. — C. Genus Gibbera: Gibbera rosae. -

(US) 38E.85. a 38E SEE", A
USOO957398OB2 (12) United States Patent (10) Patent No.: US 9,573,980 B2 Thompson et al. (45) Date of Patent: Feb. 21, 2017 (54) FUSION PROTEINS AND METHODS FOR 7.919,678 B2 4/2011 Mironov STIMULATING PLANT GROWTH, 88: R: g: Ei. al. 1 PROTECTING PLANTS FROM PATHOGENS, 3:42: ... g3 is et al. A61K 39.00 AND MMOBILIZING BACILLUS SPORES 2003/0228679 A1 12.2003 Smith et al." ON PLANT ROOTS 2004/OO77090 A1 4/2004 Short 2010/0205690 A1 8/2010 Blä sing et al. (71) Applicant: Spogen Biotech Inc., Columbia, MO 2010/0233.124 Al 9, 2010 Stewart et al. (US) 38E.85. A 38E SEE",teWart et aal. (72) Inventors: Brian Thompson, Columbia, MO (US); 5,3542011/0321197 AllA. '55.12/2011 SE",Schön et al.i. Katie Thompson, Columbia, MO (US) 2012fO259101 A1 10, 2012 Tan et al. 2012fO266327 A1 10, 2012 Sanz Molinero et al. (73) Assignee: Spogen Biotech Inc., Columbia, MO 2014/0259225 A1 9, 2014 Frank et al. US (US) FOREIGN PATENT DOCUMENTS (*) Notice: Subject to any disclaimer, the term of this CA 2146822 A1 10, 1995 patent is extended or adjusted under 35 EP O 792 363 B1 12/2003 U.S.C. 154(b) by 0 days. EP 1590466 B1 9, 2010 EP 2069504 B1 6, 2015 (21) Appl. No.: 14/213,525 WO O2/OO232 A2 1/2002 WO O306684.6 A1 8, 2003 1-1. WO 2005/028654 A1 3/2005 (22) Filed: Mar. 14, 2014 WO 2006/O12366 A2 2/2006 O O WO 2007/078127 A1 7/2007 (65) Prior Publication Data WO 2007/086898 A2 8, 2007 WO 2009037329 A2 3, 2009 US 2014/0274707 A1 Sep. -

Diseases of Trees in the Great Plains
United States Department of Agriculture Diseases of Trees in the Great Plains Forest Rocky Mountain General Technical Service Research Station Report RMRS-GTR-335 November 2016 Bergdahl, Aaron D.; Hill, Alison, tech. coords. 2016. Diseases of trees in the Great Plains. Gen. Tech. Rep. RMRS-GTR-335. Fort Collins, CO: U.S. Department of Agriculture, Forest Service, Rocky Mountain Research Station. 229 p. Abstract Hosts, distribution, symptoms and signs, disease cycle, and management strategies are described for 84 hardwood and 32 conifer diseases in 56 chapters. Color illustrations are provided to aid in accurate diagnosis. A glossary of technical terms and indexes to hosts and pathogens also are included. Keywords: Tree diseases, forest pathology, Great Plains, forest and tree health, windbreaks. Cover photos by: James A. Walla (top left), Laurie J. Stepanek (top right), David Leatherman (middle left), Aaron D. Bergdahl (middle right), James T. Blodgett (bottom left) and Laurie J. Stepanek (bottom right). To learn more about RMRS publications or search our online titles: www.fs.fed.us/rm/publications www.treesearch.fs.fed.us/ Background This technical report provides a guide to assist arborists, landowners, woody plant pest management specialists, foresters, and plant pathologists in the diagnosis and control of tree diseases encountered in the Great Plains. It contains 56 chapters on tree diseases prepared by 27 authors, and emphasizes disease situations as observed in the 10 states of the Great Plains: Colorado, Kansas, Montana, Nebraska, New Mexico, North Dakota, Oklahoma, South Dakota, Texas, and Wyoming. The need for an updated tree disease guide for the Great Plains has been recog- nized for some time and an account of the history of this publication is provided here. -

Sirex Noctilio
O R E ST E ST PE C IE S R O FIL E F P S P November 2007 Sirex noctilio Fabricius, 1793 Other scientific names: Sirex melanocerus Thomson, 1871; Paururus noctilio Order and Family: Hymenoptera: Siricidae Common names: European woodwasp; sirex; sirex woodwasp; steel-blue horntail Sirex noctilio is a major global threat to forests and the forest sector causing considerable damage and costs for control. In 1900, this pest was reported from New Zealand which represented the first time it was recorded outside of its native range. Since then it has gradually spread around the globe – to Australia in the 1960s, Latin America and the Caribbean in the 1980s, Africa in the 1990s and North America in this decade. Adult sirex woodwasps – male (L), female (R) (Photos: David R. Lance, USDA APHIS PPQ, Bugwood.org) DISTRIBUTION Native: Europe, Asia, northern Africa (Algeria, Morocco, Tunisia) Introduced: Africa: South Africa (1994) Asia and the Pacific: Australia (1961), New Zealand (1900), Tasmania (1952) Latin America and the Caribbean: Argentina (1985), Brazil (1988), Chile (2001), Uruguay (1980) North America: Canada (2005), US (2004) IDENTIFICATION Eggs are cylindrical, creamy white and approximately 0.30-0.35 mm wide and 1.35-1.56 mm long (Ciesla, 2003b). Larvae are creamy white in colour, legless with a distinctive dark spine at the posterior end (TPCP, n.d.). Length varies but larvae can reach up to 30 mm in length. Adult wasps are metallic blue-black in colour with two pairs of clear yellow membranous wings, black antennae and an upturned, spear-shaped spine or plate (cornus) at the end of the abdomen (TPCP, n.d.; W alker, 2006). -
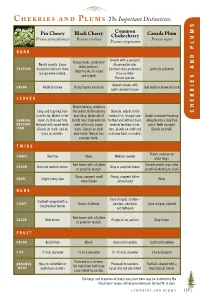
C P the Important Distinctions
C P The Important Distinctions S Common M Pin Cherry Black Cherry Chokecherry Canada Plum Prunus pennsylvanica Prunus serotina Prunus nigra U Prunus virginiana L P BARK D Smooth with a pungent, Young trunks: prominent N Nearly smooth. Large disagreeable odor. white lenticals. TEXTURE horizontal lenticels show Lenticels less prominent Lenticels yellowish A Older trunks: fissured orange when rubbed. than on other and ridged. Prunus species. S E Grayish-brown, with I COLOR Reddish-brown Young trunks are black Dull reddish-brown to black light-colored fissures R LEAVES R E Elliptic/oblong, widest in H Long and tapering from the center, thick leathery Obovate, widest in the C base to tip. Widest in the and shiny. Underside of terminal 1⁄3, sharply saw- Ovate or obovate tapering GENERAL lower 1⁄3; thin and firm midrib near stalk end cov- toothed and without hairs, abruptly into a long thin DESCRIP- textured with round teeth. ered with rusty, brown medium leathery in tex- point. Teeth rounded. TION Glands on stalk, and no hairs. Glands on stalk ture, glands on stalk and Glands on stalk. hairs on midribs. near blade. Margin has no brown hairs on midrib. rounded teeth. TWIGS Thorns common on SHAPE Very fine Waxy Medium slender older twigs Red-brown with a lighter Current growth gray, older COLOR Red and reddish-brown Gray or purplish-brown or greenish margin growth darkening to black Sharp, pungent smell Strong, pungent bitter- ODOR Slight cherry odor None when broken almond odor BUDS Cone shaped, slender – Football-shaped with a SHAPE Ovate, -

Leaf-Inhabiting Genera of the Gnomoniaceae, Diaporthales
Studies in Mycology 62 (2008) Leaf-inhabiting genera of the Gnomoniaceae, Diaporthales M.V. Sogonov, L.A. Castlebury, A.Y. Rossman, L.C. Mejía and J.F. White CBS Fungal Biodiversity Centre, Utrecht, The Netherlands An institute of the Royal Netherlands Academy of Arts and Sciences Leaf-inhabiting genera of the Gnomoniaceae, Diaporthales STUDIE S IN MYCOLOGY 62, 2008 Studies in Mycology The Studies in Mycology is an international journal which publishes systematic monographs of filamentous fungi and yeasts, and in rare occasions the proceedings of special meetings related to all fields of mycology, biotechnology, ecology, molecular biology, pathology and systematics. For instructions for authors see www.cbs.knaw.nl. EXECUTIVE EDITOR Prof. dr Robert A. Samson, CBS Fungal Biodiversity Centre, P.O. Box 85167, 3508 AD Utrecht, The Netherlands. E-mail: [email protected] LAYOUT EDITOR Marianne de Boeij, CBS Fungal Biodiversity Centre, P.O. Box 85167, 3508 AD Utrecht, The Netherlands. E-mail: [email protected] SCIENTIFIC EDITOR S Prof. dr Uwe Braun, Martin-Luther-Universität, Institut für Geobotanik und Botanischer Garten, Herbarium, Neuwerk 21, D-06099 Halle, Germany. E-mail: [email protected] Prof. dr Pedro W. Crous, CBS Fungal Biodiversity Centre, P.O. Box 85167, 3508 AD Utrecht, The Netherlands. E-mail: [email protected] Prof. dr David M. Geiser, Department of Plant Pathology, 121 Buckhout Laboratory, Pennsylvania State University, University Park, PA, U.S.A. 16802. E-mail: [email protected] Dr Lorelei L. Norvell, Pacific Northwest Mycology Service, 6720 NW Skyline Blvd, Portland, OR, U.S.A. -

Department of Environmental and Forest Biology Annual Report Summer 2016 Academic Year 2016
Department of Environmental and Forest Biology Annual Report Summer 2016 Academic Year 2016 – 2017 Donald J. Leopold Chair, Department of Environmental and Forest Biology SUNY-ESF 1 Forestry Drive Syracuse, NY 13210 Email: [email protected]; ph: (315) 470-6760 August 15, 2017 1 TABLE OF CONTENTS Introduction . .4 Overview to Annual Report . 4 Building(s) . 6 Teaching . 6 Summary of main courses taught by faculty members . .6 Course teaching load summary by faculty members . 10 Undergraduate student advising loads . 12 Curriculum changes . 12 Undergraduate students enrolled in each EFB major . 12 Listing of awards and recognition . 13 Undergraduate Recruitment Efforts . .13 Student Learning Outcomes Assessment . .14 Research/Scholarship . .14 Summary of publications/presentations . .14 Science Citation Indices . 14 Most Cited Publication of Each EFB Faculty Member . 18 Summary of grant activity . 20 Patents and Patent Applications . 22 Listing of awards and recognition . 22 Outreach and Service . 22 Service to the department, college, and university . 22 Enumeration of outreach activities . 22 Summary of grant panel service . 23 Number of journal manuscripts reviewed by faculty. 23 Summary of journal editorial board service. 23 Listing of awards and recognition . 24 Service Learning . 24 Graduate Students. .26 Number of students by degree objectives . 26 Graduate student national fellowships/awards . 26 Graduate recruitment efforts . 26 Graduate student advising . 28 2 Courses having TA support and enrollment in each . .28 Graduate Program Accomplishments – Miscellaneous. .29 Governance and Administrative Structure . .. .29 Components. .29 Supporting offices, committees, directors, and coordinators . .30 Budget . .32 State budget allocations . .32 Funds Generated by Summer Courses and Grad Tuition Incentive Program . -

THE SIRICID WOOD WASPS of CALIFORNIA (Hymenoptera: Symphyta)
Uroce r us californ ic us Nott on, f ema 1e. BULLETIN OF THE CALIFORNIA INSECT SURVEY VOLUME 6, NO. 4 THE SIRICID WOOD WASPS OF CALIFORNIA (Hymenoptera: Symphyta) BY WOODROW W. MIDDLEKAUFF (Department of Entomology and Parasitology, University of California, Berkeley) UNIVERSITY OF CALIFORNIA PRESS BERKELEY AND LOS ANGELES 1960 BULLETIN OF THE CALIFORNIA INSECT SURVEY Editors: E. G: Linsley, S. B. Freeborn, P. D. Hurd, R. L. Usinget Volume 6, No. 4, pp. 59-78, plates 4-5, frontis. Submitted by editors October 14, 1958 Issued April 22, 1960 Price, 50 cents UNIVERSITY OF CALIFORNIA PRESS BERKELEY AND LOS ANGELES CALIFORNIA CAMBRIDGE UNIVERSITY PRESS LONDON, ENGLAND PRINTED BY OFFSET IN THE UNITED STATES OF AMERICA THE SIRICID WOOD WASPS OF CALIFORNIA (Hymenoptera: Symphyta) BY WOODROW W. MIDDLEKAUFF INTRODUCTION carpeting. Their powerful mandibles can even cut through lead sheathing. The siricid wood wasps are fairly large, cylin- These insects are widely disseminated by drical insects; usually 20 mm. or more in shipments of infested lumber or timber, and length with the head, thorax, and abdomen of the adults may not emerge until several years equal width. The antennae are long and fili- have elapsed. Movement of this lumber and form, with 14 to 30 segments. The tegulae are timber tends to complicate an understanding minute. Jn the female the last segment of the of the normal distribution pattern of the spe- abdomen bears a hornlike projection called cies. the cornus (fig. 8), whose configuration is The Nearctic species in the family were useful for taxonomic purposes. This distinc- monographed by Bradley (1913). -

The Sirex Woodwasp, Sirex Noctilio: Pest in North America May Be the Ecology, Potential Impact, and Management in the Southeastern U.S
SREF-FH-003 June 2016 woodwasp has not become a major The Sirex woodwasp, Sirex noctilio: pest in North America may be the Ecology, Potential Impact, and Management in the Southeastern U.S. many insects that are competitors or natural enemies. Some of these insects compete for resources AUTHORED BY: LAUREL J. HAAVIK AND DAVID R. COYLE (e.g. native woodwasps, bark and ambrosia beetles, and longhorned beetles) while others (e.g.parasitoids) are natural enemies and use Sirex woodwasp larvae as hosts. However, should the Sirex woodwasp arrive in the southeastern U.S., with its abundant pine plantations and areas of natural pine, this insect could easily be a major pest for the region. Researchers have monitored and tracked Sirex woodwasp populations since its discovery in North America. The most common detection tool is a flight intercept trap (Fig. 2a) baited with a synthetic chemical lure that consists of pine scents (70% α-pinene, 30% β-pinene) or actual pine branches (Fig. 2b). Woodwasps are attracted to the odors given off by the lure or cut pine branches, and as they fly toward the scent they collide with the sides of the trap and drop Figure 1. The high density of likely or confirmed pine (Pinus spp.) hosts of the Sirex woodwasp suggests the southeastern U.S. may be heavily impacted should this non-native insect become into the collection cup at the bottom. established in this region. The collection cup is usually filled with a liquid (e.g. propylene glycol) that acts as both a killing agent and Overview and Detection preservative that holds the insects until they are collected. -

Sand Plums for Home and Commercial Production
Oklahoma Cooperative Extension Service HLA-6258 Sand Plums for Home and Commercial Production Beth McMahon Oklahoma Cooperative Extension Fact Sheets Research Assistant Oklahoma State University are also available on our website at: http://osufacts.okstate.edu Bruce Dunn Assistant Professor Geyer, 2010). Flowering will last for a couple of weeks and Oklahoma State University either red or yellow fruit will begin to form afterward. Ripening of the fruit occurs from June to early August and are either Sand plums, also known as Chickasaw plum, Cherokee yellow or a bright red. Both colors occur in the same areas plum, or Sandhill plum (Prunus angustifolia Marshall), are native of Oklahoma. Fruit size can range from ¼ inch to 1 inch. It fruit-producing shrubs or small trees in Oklahoma (Figure 1). is recommended that long sleeves be worn while collecting Use of sand plums range from cover for native bird species fruit since the plants may be thorny, depending upon how to making jams, jellies, and wine from the fruit. Commercial damaged they have been by deer and cattle in the past. desire in making jams and jellies has led to a rising interest in cultivating sand plums for home and orchard production. The purpose of this publication is to provide some basic knowledge Selecting Plants on how to identify, propagate, and grow your own sand plums. Besides selecting plants for fruit size and crop load, Sand plums range from 2 feet to 25 feet high, depend- you may also want to consider selecting plants that have ing upon soil and water conditions (Row and Geyer, 2010). -

Kentucky Fruit Facts
Kentucky Fruit Facts January-February Newsletter 2018 http://www.uky.edu/hort/documents-list-fruit-facts Cooperative Extension Service John Strang, Extension Fruit Specialist, Editor University of Kentucky Horticulture Department Denise Stephens, Newsletter Designer 1100 So. Limestone St. Lexington Ky 40546-0091 (859) 257-2909 Inside this Issue: Fax: (859) 257-2859 Fruit Crop News . .1 extension.ca.uky.edu Upcoming Meetings. .2 Thornless Erect Blackberry Cultivar Trial . 3 of the state showing that we had a few spots that Pesticide Certification: Who Needs It? . .4 were particularly cold and peach flower bud damage Black Knot. .6 probably occurred in these areas. The temperatures Cane Diseases of Brambles. .6 below 0°F more than likely caused damage to Receiving Fruit Facts on the Internet . .8 thornless blackberries. The rule of thumb is that for every degree below 0°F thornless blackberries lose 10% of the crop. Keep in mind that these Fruit Crop News low temperatures occurred in early January when John Strang, U.K. Extension Horticulturist and Matt Dixon, blackberries were at their maximum hardiness level U.K. Ag Meteorologist so there may not be any injury to plants where the temperature was a few degrees below 0°F. We are hopefully through the coldest portion While pruning watch for signs of San Jose of the winter and it is time to start pruning apple and Scale in your trees. Feeding by each insect causes pear trees. Prune the oldest trees first leaving your an increase in the reddish purple pigments beneath youngest until later in the spring. If you have semi- the bark leaving a small circular spot around the dwarf or dwarf trees that are spaced out and trained to feeding site.