Effect of Rovatirelin in Patients with Cerebellar Ataxia: Two Randomised
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Hopeful Future for Japan
As of December 20, 2017 Hopeful future for Japan Nippon Healthcare Investment Corporation Presentation Material for The 7th Fiscal Period Ended October 2017 Securities code : 3308 Contents Disclaimer Continuous Evaluation of Operators and Monitoring System 19 Highlights of 7th Fiscal Period (Ended October 2017) 3 Implementation of Monitoring and Section 1 Relationships with Operators 20 Operating Results for 7th Fiscal Period Introduction of Operators 21 Operator Initiatives (AI and System Introduction) 22 Operating Results for 7th Fiscal Period 5 Forecast of Operating Results for 8th Fiscal Period Section 4 and 9th Fiscal Period 6 Financial Status Distribution per Unit 7 Status of Interest-Bearing Liabilities Section 2 (as of October 31, 2017)・・・・・・・・・・・・・・・・・・・・ 24 External Growth Policy on Distributions in Excess of Earnings/Index including NHI 25 Change in Asset Size 9 Overview of the Unitholder Benefits Program・・・・・・・・・・・・・ 26 Sourcing Route 10 ・・・・・・・・・・・・・・ Section 3 Section 5 Portfolio Analysis Environment Surrounding Healthcare Industry Portfolio List (as of October, 2017) 12 Surrounding Status of Healthcare Facilities : Status of Portfolio: Change in Occupancy Rates 13 Progress of aging society 29 Remaining Lease Contract Period of Each Property 14 Market Size by Segment( as of June 2016) 30 Status of Portfolio: Fee Systems of Facilities 15 History of Revisions to Nursing Care Fees 31 Status of Portfolio (as of October 31, 2017) 16 Portfolio Resident Attributes (as of October 31, 2017) 17 Appendix Properties Renovated in the 7th Fiscal Period 18 This document is not material disclosed in compliance with the Financial Instruments and Exchange Act or any regulation of similar nature, nor is it intended for the purposes of solicitation. -

Subject Index
Subject Index Aalto, Alvar, 22 Art Shed Southbank Architectural Com- bearing, solar angle diagrams, 334 Ackerman Student Union, University of petition, 2006, 383 Berkeley Art Museum and Pacific Film California at Los Angeles, Cali- Asia Society, Hong Kong Center, China, Archive, Berkeley, California, fornia, 250 410, 411 197 Adelaide University, South Australia, assembly drawing, linear perspective Bernal Heights residence, San Fran- School of Architecture, Land- drawing, 311 cisco, California, 262 scape Architecture and Urban Atocha Station, Madrid, Spain, 75 B FIVE STUDIO, 550 Design, 499 audience, portfolio, 463–64 Bilbao Effect, 120 Adelman/Liano residence, Santa Moni- August Wilson Center for African Ameri- BIM (building information modeling), ca, California, 207 can Culture, Pittsburgh, Penn- capabilities of, 93–94 Adobe Photoshop, 63 sylvania, 284 bird’s eye perspective African American Museum of Slavery, Aulenti, Gaetana, 22 linear perspective drawing, 246–47, Rockville, Maryland, 429 automobile 298–301 Aga Han Museum, Toronto, Canada, conceptual sketching, 73 one-point perspective from above, 242 representational drawing, 26–29 linear perspective drawing, Alaska, University of, Fairbanks, 198 auxiliary views, multiview-drawings, 177 248–49 alternatives, conceptual diagramming axes addition, orthographic and paraline underside perspective with, linear and sketches, 40, 44–45 drawing, 195 perspective drawing, 303 altitude, solar angle diagrams, 334 axial lines, orthographic and paraline Birkerts, Gunnar, 42, 69, 150 American Museum -
Guia2-Oriente.Pdf
Facultad de Arquitectura UDELAR Montevideo | Uruguay GRUPO DE VIAJE 2013 ARQUITECTURA RIFA G06 EQUIPO DOCENTE Adriana Barreiro Jorge Casaravilla Gustavo Hiriart Pablo Kelbauskas Bernardo Martín Ximena Rodríguez Soledad Patiño Ernesto Spósito MÓDULO 02 ORIENTE DOCENTES MÓDULO 02 Adriana Barreiro Ernesto Spósito Ximena Rodríguez Nota importante: Las Guías de los Grupos de Viaje de la Facultad de Arquitectura de la Universidad de la República son el resultado del trabajo de sucesivos Equipos Docentes Directores y generaciones de estudiantes. En particular, el material contenido en las presentes Guías fue compilado por el Grupo de Viaje Generación 2005 y su Equipo Docente Director del Taller Danza, quienes realizaron su viaje de estudios en el año 2012. Este material ha sido editado y adaptado al proyecto académico del Grupo de Viaje Generación 2006, cuyo viaje de estudios se realizará en el año 2013. Facultad de Arquitectura UDELAR GRUPO DE VIAJE 2012 ARQUITECTURA RIFA G05 EQUIPO DOCENTE Taller Danza Marcelo Danza Lucía Bogliaccini Luis Bogliaccini Diego Capandeguy Marcos Castaings Martín Delgado Andrés Gobba Lucas Mateo Nicolás Newton Natalia Olivera Felipe Reyno Thomas Sprechmann Marcelo Staricco MÓDULO 02 ORIENTE DOCENTES MÓDULO 02 Tomás Sprechman Diego Capandeguy Nicolás Newton GRUPO DE TRABAJO Natalie Cordero Mariana García Etcheverry Bruno La Buonora Magdalena Ponce de León Katia Sei Fong Santiago Serrano Sofía Damiani Mariano García Patricia Izaurralde JAPÓN DatoS GENERALES: Superficie: 37.800 km2. (Con menos del 7% del suelo urbanizable) Población: 128 .100.000 (2010). (Es la décima más grande del mundo) Densidad de Población: 3.336 habitantes por km2 Territorio: Archipiélago, con cuatro islas principales que forman el 97% de la superficie total del país, y con 6.848 islas menores adyacentes. -

English Program, Friday, February 15
The 49th Annual Meeting of the Japanese Society for Replacement Arthroplasty Feb.15(Fri.) Feb.15(Fri.) Day 1 Program of the 49th Annual Meeting of Day 1 the Japanese Society for Replacement Arthroplasty Day 2 Feb.16(Sat.) Friday, February 15, Room 1 8:00~8:10 Opening Ceremony Room 1 Room 1 8:10~9:40 Panel Discussion 1 Implant designs and biomechanics of TKA Room 2 Moderators:Hiromasa…MIURA,…Shuichi…MATSUDA 1-1-PD1-01 Effect…of…implants…design…on…in…vivo…kinematics…after…total…knee…arthroplasty Dept. of Orthop. Biomaterial Sci., Osaka Univ. Tetsuya TOMITA, et al ...... 292 Room 3 1-1-PD1-02 Improvement…of…TKA…design…based…on…kinematic…analysis Second Dept. of Orthop. Surg., Dokkyo Medical Univ. Saitama Medical Center/ Dept. of Orthop. Surg., Medical Hospital, Room 4 Tokyo Medical and Dental Univ. Toshifumi WATANABE, et al ...... 292 1-1-PD1-03 TKA…design…from…the…point…of…view…of…3D…morphology…and…kinematics Dept. of Orthop. Surg., Niigata Medical Center Takashi SATO, et al ...... 293 Room 5 1-1-PD1-04 Design…and…biomechanics…of…posterior…stabilized…TKA Dept. of Orthop. Surg., Osaka City Univ. Yukihide MINODA, et al ...... 293 1-1-PD1-05 Total…Knee…Artoroplasty…Design…based…on…Computer…Simulation…analyses Room 6 Dept. of Orthop. Surg., Kyoto Univ. Shinichi KURIYAMA, et al ...... 294 1-1-PD1-06 TKA…design…-Effect…evaluation…and…development…of…next…generation…TKA- Dept. of Orthop. Surg., Ehime Univ. Kazunori HINO, et al ...... 294 Room 7 9:45~11:15 Panel Discussion 2 The facts of THA surgery on the basis of spino-pelvic alignment Room 8 Moderators:Masaaki…MAWATARI,…Yutaka…INABA 1-1-PD2-01 Stress…distribution…for…asetabulum…according…with…the…alignment…of…pelvis…-…spine Faculty of Health and Sport Sciences, Room 9 University of Tsukuba Shumpei MIYAKAWA, et al ..... -

Watanabe, Tokyo, E
Edition Axel Menges GmbH Esslinger Straße 24 D-70736 Stuttgart-Fellbach tel. +49-711-574759 fax +49-711-574784 Hiroshi Watanabe The Architecture of Tokyo 348 pp. with 330 ill., 161,5 x 222 mm, soft-cover, English ISBN 3-930698-93-5 Euro 36.00, sfr 62.00, £ 24.00, US $ 42.00, $A 68.00 The Tokyo region is the most populous metropolitan area in the world and a place of extraordinary vitality. The political, economic and cultural centre of Japan, Tokyo also exerts an enormous inter- national influence. In fact the region has been pivotal to the nation’s affairs for centuries. Its sheer size, its concentration of resources and institutions and its long history have produced buildings of many different types from many different eras. Distributors This is the first guide to introduce in one volume the architec- ture of the Tokyo region, encompassing Tokyo proper and adja- Brockhaus Commission cent prefectures, in all its remarkable variety. The buildings are pre- Kreidlerstraße 9 sented chronologically and grouped into six periods: the medieval D-70806 Kornwestheim period (1185–1600), the Edo period (1600–1868), the Meiji period Germany (1868–1912), the Taisho and early Showa period (1912–1945), the tel. +49-7154-1327-33 postwar reconstruction period (1945–1970) and the contemporary fax +49-7154-1327-13 period (1970 until today). This comprehensive coverage permits [email protected] those interested in Japanese architecture or culture to focus on a particular era or to examine buildings within a larger temporal Buchzentrum AG framework. A concise discussion of the history of the region and Industriestraße Ost 10 the architecture of Japan develops a context within which the indi- CH-4614 Hägendorf vidual works may be viewed. -
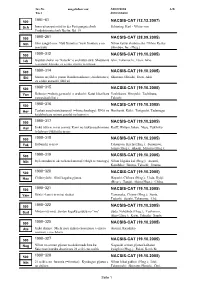
1991-61 Nacsis-Cat
Inv.Nr. ausgeliehen von: Anmerkung A/B Titel Autorenname 1991-61 500 NACSIS-CAT (12.12.2007) Sch Innovationspotential in der Fertigungstechnik Schöning, Karl - Viktor von Produktionstechnik Berlin; Bd. 19 1990-261 500 NACSIS-CAT (28.09.2005) Nih Shin sangyô ron: Nyû furontia (=new frontier) e no Nihon keizai shinbun sha / Nihon Keizai senryaku Shimbun, Inc. (Hrsg.) 1990-312 500 NACSIS-CAT (19.10.2005) Ish Gijutsu shakai no "katachi" o enshutsu suru: Shisutemu Ishii, Takemochi; Etori, Akio (=system) kôgaku ga egaku gijutsu to ningen 1990-314 500 NACSIS-CAT (19.10.2005) Shi Seimei ni jôhô o yomu: Baiohoronikusu (=bioholonics) Shimuzu, Hiroshi; Etori, Akio ga egaku atarashii jôhô zô 1990-315 500 NACSIS-CAT (19.10.2005) Yos Robotto (=robot) ga machi o aruku hi: Katai kikai kara Yoshikawa, Hiroyuki; Tachibana, yawarakai kikai e Takashi 1990-316 500 NACSIS-CAT (19.10.2005) Hor Tenkan suru baiotekunorojî (=biotechnology): DNA no Horikoshi, Kôki; Taniguchi, Tadatsugu kaidoku kara seimei genshô no kaimei e 1990-317 500 NACSIS-CAT (19.10.2005) Kol Jinkô zôki ni mirai o miru: Kami no tsukurareshi mono Kolff, Willem Johan; Nose, Yukihiko to hito no tsukurishi mono 1990-318 500 NACSIS-CAT (19.10.2005) Tak Kôbunshi to iryô Takemoto, Kiichi (Hrsg.); Sunamoto, Junzô (Hrsg.); Akashi, Mitsuru (Hrsg.); 1990-319 Tsuruta, Teiji; Imanishi, Yukio; Gen, 500 NACSIS-CAT (19.10.2005) Nih Iryô shindan ni okeru haitekunorojî (=high technology) Nihon kôgaku kai (Hrsg.); Atsumi, Kazuhiko; Iinuma, Takeshi; Iinuma, 1990-320 Kazuhiro; Tateno, Yukio; 500 NACSIS-CAT (19.10.2005) -

Litteraturliste
LITTERATURLISTE „Aoyama Technical College“ in Architectural 1996, pp. 110-27. „From a Talk with Hiroshi Hara“ in Mitsumasa, Hara, H.: „Architecture and Individuality“ in Review 1136 okt. 1991, pp. 45-49. Choudhury, S. & Clavijo, C.: „Machiya. A Survey F. (Ed.): Architecture Riffle 006: Umeda Japan Architect 6/1966, pp. 87-107. „Aoyama Technical College“ in Japan Architect of Traditional Japanese Urban Houses“ in Sky Building Sky Building, Toto, Tokyo Hara, H.: „500Mx500Mx500M Cube“ in GA Ar- 2/1991, pp. 160-63. Japan Architect 1/1972, pp. 91-104. 1993. chitect 13: Hiroshi Hara, A.D.A. Edita, „Amusement Complex <H>“ in Japan Archi- Cooper, M. (overs. & Ed.): This Island of Ja- GA Architect 9: Shin Takamatsu, A.D.A. Edita, Tokyo 1990, pp. 240-43. tect 1/1994, pp. 170-73. pan. João Rodrigues’ Account of 16th- Tokyo 1990. Hara, H.: „On the ‘Drifting Space’ and the Ar- Bellini, M.: „Architecture and Cities“ in Japan century Japon, Kodansha International, GA Architect 13: Hiroshi Hara, A.D.A. Edita, chitectural Forms“ in Japan Architect Architect 3/1992, pp. 172-79 Tokyo & New York 1973. Tokyo 1990. 3/1993, pp. 26-31. Bellini, M.: „Tokyo Design Center“ in Japan Davies, C.: „Office Interiors“ in Davies, C. & Gion. Suina asobi no sekai, (Gion. Den ele- Hara, H.: „Extraterrestrial Architecture Project“ Architect 3/1992, pp. 180-91 Lambot I.: Century Tower, Ernst & Sohn, gante legeverden, ISBN4-473-01399-5) in GA Architect 13: Hiroshi Hara, A.D.A. Bognar, B.: „From Ritualistic Objects to Science Berlin 1992, pp. 141-59. Hakoosho, Kyoto 1995. Edita, Tokyo 1990, pp. -

Vas-Cog Journaljournal
JAPANESESOCIETYOFVASCULARCOGNITIVEIMPAIRMENT(2018NO.4) Vas-CogVas-Cog JournalJournal No.4 Apr 001.1. 2018 Official Journal of Vas-Cog Japan Society 1 ISSN:24239372 JAPANESESOCIETYOFVASCULARCOGNITIVEIMPAIRMENT(2018NO.4) Greetings from Vas-Cog Japan, the Japanese Society for Vascular Cognitive Impairment Ryuichi Morishita, MD, PhD President:TheJapaneseSocietyforVascularCognitiveImpairment IamDr.RyuichiMorishitafromOsakaUniversity, programatagreatvenueorganizedbythechairmen. andItookoverasPresidentoftheJapaneseSociety The9thannualmeetingistobeheldatBeppu forVascularCognitiveImpairmentfromDr.KojiAbe. InternationalConventionCenterAugust4-5,2018, Iwouldliketofurtherdevelopthissociety-which withDr.KatsuyaUrakamiandDr.EtsuroMatsubara wasfirstdevelopedbyDr.Nagata,thefirstPresident, aschairmen,soweareexpectingmanyofyouto andDr.Abe,thesecondPresident-tothebestofmy attend. ability,withthecooperationofallthemembersin OuraffiliateVas-CogAsiahasbeenrevitalizing oursociety,soyourkindsupportwouldbegreatly yearbyyear.The6thmeetingheldinNanjingCity appreciated. onOctober26,2017,wasahugesuccesswithVas- Vas-CogJapan,whichisinitseighthyearfromits CogAsiaDirector,Dr.KojiAbeandDr.Shinichiro timeasastudygroup,hasdevelopedquite Uchiyamaservingaslecturersandmoderators. The considerably. Asoursocietyhasalreadymetthe 7thannualmeetingwillbeheldinJakartaon requirementstoberegisteredasanacademic September6,2018.Vas-CogWorldwillalsobeheld organizationintheScienceCouncilofJapanwith inHongKongNovember24-27,2018. regardtothenumberofmembers,membership WithregardtothemajordementiassuchasLewy -

AHST6333-FA13 Syllabus
AHST6333 FA13 Urban Morphology AHST6333: Architectural History/Theory Seminar The Urban Morphology of Kyoto and New Orleans Instructors: Kentaro Tsubaki / TBD Introduction: Japanophile, spent 10 years as a journalist in New Or- leans prior to moving to Japan at the age of 40 never to “Like many of the Creole houses, the facade presented a return. He dedicates the rest of his life drawing awareness commonplace and unattractive aspect. The great green to the beauty and tranquility of pleasing customs and last- doors of the arched entrance were closed; and the green ing values of his adopted country at odds with the emerg- shutters of the balconied windows were half shut, like ing western style materialism. sleepy eyes lazily gazing upon the busy street below or the cottony patches of light clouds which floated slowly, Ignoring the obvious nouns identified with the location and slowly across the deep blue of the sky above. But beyond concentrating on the spatial relationships of Hearn’s re- the gates lay a little Paradise. The great court, deep and marks, the historical and geographical boundary becomes broad, was framed in tropical green; vines embraced the a blur, infinitely difficult to distinguish, transcending time white pillars of the piazza, and creeping plants climbed up and place. The goal of this study abroad studio is to re- the tinted walls to peer into the upper windows with their contextualize the familiar by dislocating to the unfamiliar. flower-eyes of flaming scarlet.” Kyoto is a city steeped in tradition and cultural heritage Lafacdio Hearn, “Creole Court, Leaves” From The Diary of an Impression- outside of the typical western society. -

ME Week Program E Regular 20120210Web
ME Week 2012 in Chiba - Program - 21 Feb. 2012 (Tuesday) 10:00 - 11:00 ME Week 2012 in Chiba Opening Ceremony Koichi ITO (Director of Research Center for Welcome Address Frontier Medical Engineering, Chiba University) Opening Remarks Yasushi SAITO (President of Chiba University) Kaname TAJIMA (Member of the House of Messages from Guests Representatives, Japan) Toshihito KUMAGAI (Mayor of Chiba City, Japan) Hisashi KATO (Director of International Program Department, Japan Sosiety for the Promotion Science) Shigeto ODA (Director of Deparment of Emergency and Critical Care Medicine / Vice Director of Research Center for Frontier Medical Engineering, Japan) An Introduction of Medical Engineering in Chiba Koichi ITO (Director of Research Center for University Frontier Medical Engineering, Chiba University) 11:00 - 12:00 Keynote Talk I "Translational Research Concept in Biomedical Engineering" Lecturer: Kerong DAI (Academician of China Engineering Academy, Shanghai JiaoTong University, Shanghai Ninth People’s Hospital, China) Chair: Kazuhisa TAKAHASHI (Chiba University, Japan) 12:00 - 13:00 Lunch 13:00 - 15:15 3T-in-3A 2012 and MECU 2012 Joint Sessions "Clinically-Driven Development of IT for Diagnosis and Bio-Modelling" Chair: See Kiong NG (Singapore University of Technology and Design, Singapore) Leading 13:00 - 13:35 L1-1: Fusion and Enrichment of Medical Imaging for High Hideaki HANEISHI (Chiba University, Japan) Quality Diagnosis and Treatment (FERMI) Clinically-Driven Research and Digital Technology Dongyun GU (Shanghai Jiao Tong University, -
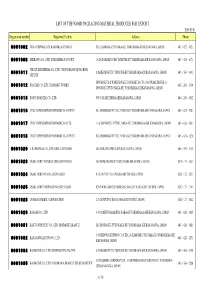
LIST of the WOOD PACKAGING MATERIAL PRODUCER for EXPORT 2005/03/01 Registration Number Registered Facility Address Phone
LIST OF THE WOOD PACKAGING MATERIAL PRODUCER FOR EXPORT 2005/03/01 Registration number Registered Facility Address Phone 0001002 ITOS CORPORATION KAMOME-JIGYOSHO 62-1 KAMOME-CHO NAKA-KU YOKOHAMA-SHI KANAGAWA, JAPAN 045‐622‐1421 0001008 ISHIKAWA CO., LTD. YOKOHAMA FACTORY 18-24 DAIKOKU-CHO TSURUMI-KU YOKOHAMA-SHI KANAGAWA, JAPAN 045‐521‐6171 THE IZUMI EXPRESS CO., LTD. TOKYO BRANCH, PACKING 0001011 8 DAIKOKU-FUTO TSURUMI-KU YOKOHAMA-SHI KANAGAWA, JAPAN 045‐504‐9431 CENTER HONMOKU B-2 WARE HOUSE, HONMOKU D-CFS 1 GO WARE HOUSE 3-1 0001012 INAGAKI CO., LTD. HONMOKU WORKS 045‐260‐1160 HONMOKU-FUTO NAKA-KU YOKOHAMA-SHI KANAGAWA, JAPAN 0001013 INOUE MOKUZAI CO., LTD. 895-3 SYAKE EBINA-SHI KANAGAWA, JAPAN 046‐236‐6512 0001016 UTOC CORPORATION HONMOKU B-1 OFFICE B-1, HONMOKU-FUTOU, NAKA-KU, YOKOHAMA-SHI, KANAGAWA, JAPAN 045‐621‐5781 0001017 UTOC CORPORATION HONMOKU D-5 CFS 1-16, HONMOKU-FUTOU, NAKA-KU, YOKOHAMA-SHI, KANAGAWA, JAPAN 045‐623‐1241 0001018 UTOC CORPORATION HONMOKU B-3 OFFICE B-3, HONMOKU-FUTOU, NAKA-KU, YOKOHAMA-SHI, KANAGAWA, JAPAN 045‐621‐6226 0001020 A.B. SHOHKAI CO., LTD. EBINA-JIGYOSHO 642 NAKANO EBINA-SHI KANAGAWA, JAPAN 046‐239‐0133 0001023 OSAKI CORP. TATEBAYASHI EIGYOUSHO 358 NOBE-MACHI TATEBAYASHI-SHI GUNMA, JAPAN 0276‐74‐6531 0001024 OSAKI CORP. OYAMA EIGYOUSHO 4-18-39 JYOUTOU OYAMA-SHI TOCHIGI, JAPAN 0285‐22‐1211 0001025 OSAKI CORP. NISHINASUNO EIGYOUSHO 429-9 NIKU-MACHI NISHINASUNO-CHO NASU-GUN TOCHIGI, JAPAN 0287‐37‐7161 0001028 OYAMA LUMBER CORPORATION 2-7-26 TENJIN-CHO OYAMA-SHI TOCHIGI, JAPAN 0285‐22‐0022 0001029 KAGAMI CO., LTD. -

The Vitality and Resilience of Inherited Japanese Houses -100 Years of Shimizu-Gumi Houses-
The Vitality and Resilience of Inherited Japanese Houses -100 Years of Shimizu-gumi Houses- Preface Since its foundation in 1804, residential architecture had been one of the primary business focuses for Shimizu-gumi, the present Shimizu Corporation. Many books have been published to showcase its works, such as Sekkei zushū, jūtaku no maki, ji 1907 nen shi 1923 nen (Drawing collection: house, from 1907 to 1923) and Sekkei zushū, shitsunai narabini kagu dentō no maki, ji 1909 nen shi 1913 nen (Drawing collection: interior, furniture and lighting, from 1909 to 1913). These books feature painted drawings of large houses, which could be considered mansions in the Western sense, designed and built by Shimizu-gumi, and include plans, elevations, development plans, and illustrations of furniture. The books enable the reader to visualize many aspects of mansions in the Meiji era (1868-1912) and Taisho era (1912-1926,) and to understand how these mansions were significant as elements of urban culture. Additionally, an academic work based on these earlier texts was published to commemorate the 60th anniversary of the Housing Research Foundation JUSOKEN: Meiji/Taisho no teitaku, Shimizu-gumi sakusei saishiki-zu no sekai (Mansions in Meiji and Taisho, the world of colored drawings created by Shimizu-gumi, Kashiwashobo, 2009, currently unavailable). This book is the product of joint research by “Shimizu Kensetsu Teitaku Shiryō Kenkyūkai” (Shimizu Corporation Mansion Document Study Group, 2004 to 2009) in the “Jūtaku Shiryō Iinkai” (Committee for historical materials about houses) of Jusoken. For the next phase of study, surveys and research on Jūtaku kenchiku zushū (Residential architecture catalog, 1st volume: 1935, 2nd volume: 1939) should be conducted.